Abstract
The aim of this study was to investigate the potential protective effect of thymoquinone against cisplatin-induced ototoxicity. This study is a prospective, controlled experimental animal study. Experiments were performed on 30 healthy female Sprague-Dawley rats. Thirty animals were divided into three groups of 10 animals each. Group 1 received an intraperitoneal (i.p.) injection of cisplatin 15 mg/kg. Group 2 received i.p. thymoquinone 40 mg/kg/day for 2 days prior to cisplatin injection and third day i.p. cisplatin 15 mg/kg was administered concomitantly. Group 2 continued to receive i.p. thymoquinone until fifth day. Group 3 received i.p. thymoquinone 40 mg/kg/day for 5 days. Pretreatment distortion product otoacoustic emissions (DPOAE) and auditory brain stem responses (ABR) testing from both ears were obtained from the animals in all groups. After the baseline measurements, drugs were injected intraperitonally. After an observation period of 3 days, DPOAE measurements and ABR testing were obtained again and compared with the pretreatment values. There was no statistically significant difference between pre and post-treatment DPOAE responses and ABR thresholds group 2 and 3. However, group 1 demonstrated significant deterioration of the ABR thresholds and DPOAE responses. Our results suggest that DPOAE responses and ABR thresholds were preserved in the cisplatin plus TQ-treated group when compared with the group receiving cisplatin alone. According to these results, cisplatin-induced ototoxicity may be prevented by thymoquinone use in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is commonly used as an antineoplastic agent to treat a range of several neoplastic diseases including ovarian, testicular, bladder, lung, head and neck [1]. The antineoplastic action mechanism is associated to the inhibition of the deoxyribonucleic acid (DNA) synthesis [2]. Dose-limiting side effects of cisplatin are nephrotoxicity, neurotoxicity and ototoxicity [3]. Ototoxicity may occur within hours to days after cisplatin application [1]. Cisplatin-induced ototoxicity is usually bilateral, progressive, irreversible and sensorineural hearing loss initially at high frequencies. Hearing impairment may progress to involve all frequencies when high cumulative dose cisplatin is used [1].
The exact mechanisms of cisplatin-induced ototoxicity are still not well understood. The excessive production of free oxygen radicals in the cochlear tissues is the most popular mechanism underlying cisplatin ototoxicity [1]. Cisplatin also decreases antioxidant enzymes in the cochlea [4]. Once the stability between free oxygen radicals production and the antioxidative defiance mechanisms is impaired, oxidative stress can occur, which can result in cochlear cell injury or death.
Cisplatin-induced ototoxicity has been demonstrated in experimental animal studies and many studies have reported on the protective effects of various antioxidant agents such as resveratrol, melatonin, dexamethasone, Vitamine E, d-methionine, N-acetylcysteine, gingko biloba extract, allopurinol and ebselen, amiphostine, sodium salicylate, Salvia miltiorrhiza and pomegranate extract [4–16].
Thymoquinone (TQ) is the main constituent of the volatile oil of the Nigella sativa (black seed). TQ has many therapeutic effects including analgesic, antiinflammatory, antibacterial, antidiabetic, antiulcerogenic, antineoplastic and immunomodulatory [17]. The antioxidant effect of TQ is considered to be one of its most significant properties [18]. It has been reported that TQ neutralizes free oxygen radicals acting as a cleaner and increases the level of antioxidant enzymes [19]. In addition, previous studies demonstrated that TQ might have a protective effect against cardiotoxicity, hepatotoxicity and nephrotoxicity induced by various agents. These agents include the doxorubicine-induced cardiotoxicity, carbon tetrachloride-induced hepatotoxicity, vancomycin and cisplatin-induced nephrotoxicity [18, 20–22]. However, the protective effect of TQ on the cisplatin-induced ototoxicity has not been investigated. The aim of this study was to investigate the potential protective effect of TQ against cisplatin-induced ototoxicity.
Materials and methods
The protocol of this study was approved by the Ethical Committee on Animal Research of Erciyes University (2012-08). The study was done at the Experimental Animals Studies Laboratory of Erciyes University.
Animals
In this study, 30 healthy female Sprague-Dawley rats, weighing 200–270 g and averaging 24 weeks old were utilized. The animals were kept in separate cages in a temperature controlled room that maintained a 12-h light/dark cycle with free reach to food and water. At the beginning of the study, we examined the external ear canal and the tympanic membrane of each rat with an operating ear microscope. Any rat with external ear infection, serous otitis media or tympanic membrane perforation ear was excluded from this study.
Experimental groups
Thirty animals were divided into three groups of 10 animals each and treated as follows: group 1 received an intraperitoneal (i.p.) injection of cisplatin 15 mg/kg (Cisplatin-Hospira 100 mg/100 ml; UK). Group 2 received i.p. thymoquinone 40 mg/kg/day (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) for 2 days prior to cisplatin injection and third day i.p. cisplatin 15 mg/kg was administered concomitantly. Group 2 continued to receive i.p. thymoquinone until fifth day. Group 3 received i.p. thymoquinone 40 mg/kg/day for 5 days.
Study design
Animals were intraperitoneally anesthetized with 100 mg/kg ketamine and 7.5 mg/kg xylazine. Under general anesthesia, after ear microscopic examination, pretreatment DPOAE measurements and ABR testing from both ears were obtained from the animals in all groups. After the baseline measurements, drugs were injected intraperitonally. After an observation period of 3 days, DPOAE measurements and ABR testing were obtained again and compared with the pretreatment values.
Auditory tests
The hearing was assessed by DPOAE and ABR under general anesthesia. All measurements were performed in a quiet room.
DPOAE measurements
Otodynamics ILO-288 Echoport equipment (Otodynamics Ltd., London, UK) was used to measure DPOAEs. An infant hearing screening probe was placed to the external ear canal and the measurements were performed. The sound stimulus that composed DPOAEs consisted of two simultaneous permanent pure tones at different frequencies (f 1/f 2 ratio = 1.22) at 80 dB SPL (L1 = L2). DPOAEs were measured at seven different frequencies ranged from 1,000 to 8,000 Hz (1,001, 1,501, 2,002, 3,003, 4,004, 6,006, 7,996).
ABR measurements
The ABR test was performed on both ears of rats and the records were acquired through two channels. We used an interacustic EP25 instrument and ABR 3A ear phone. The responses were recorded using subdermal needle electrodes. The active electrode was placed at the vertex, the reference electrodes were placed in the right and left mastoid regions and the graund electrode was placed on the glabella. The ABR test was done by 1,000 click stimulus at a rate of 21 times per second. Band-pass filters 100–3,000 Hz for click stimulus. Measurements were obtained at 70 dBnHL and decreased by increments of 20 dB until the threshold was approached. Repeatibility was confirmed and the threshold determination was developed by testing twice. ABR threshold was defined on the fifth wave.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software package (SPSS, version 16.0 for windows; SPSS Inc., Chicago, IL, USA). All data were presented as mean ± SD. Comparison among the groups with DPOAE values and ABR thresholds were evaluated using one-way analysis of variance (ANOVA) with the Bonferroni post- hoc test. Paired samples t test was used to compare the DPOAE values and ABR thresholds before and after drug application in each group. Differences were considered statistically significant at p value of <0.05.
Results
One animal from the cisplatin group died under anesthesia during study and 29 animals completed the study without any complications. Hearing assessment was performed for 58 ears of 29 rats.
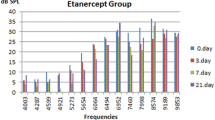
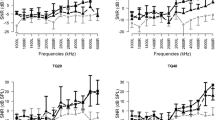
When pretreatment DPOAE values were compared between the groups, there was no significant difference among all groups in all frequencies. Post-treatment DPOAE responses were found to be lower than pretreatment DPOAE responses in group 1 and the differences were statistically significant for 2,000, 3,000, 4,000, 6,000 and 8,000 Hz (Fig. 1). However, there was no statistically significant difference between the pre and post-treatment DPOAE responses for all tested frequencies of the group 2 and 3 (Figs. 2, 3).
Table 1 demonstrates the ABR thresholds for click stimuli before and after drug application for all groups. When pretreatment ABR thresholds were compared between the groups, there was no significant difference between the groups. There was no statistically significant difference between the pre and post-treatment mean ABR thresholds of the group 2 and 3. An example of ABR recordings before and after cisplatin plus TQ application is demonstrated in Fig. 4. The difference in mean ABR thresholds after cisplatin administration was statistically significant (Table 1). An example of ABR recordings before and after cisplatin administration is demonstrated in Fig. 5.
When post-treatment DPOAE values and ABR thresholds were compared between the groups, there were statistically significant differences DPOAE values for 2,000, 3,000, 4,000, 6,000 and 8,000 Hz and ABR thresholds. DPOAE values for 2,000, 3,000, 4,000, 6,000 and 8,000 Hz in the cisplatin group were significantly decreased compared to other groups (p < 0.05); also, ABR thresholds were significantly increased in the cisplatin group compared to other groups (p < 0.001) (table 1). There were no statistically significant differences between groups with post-treatment DPOAE values for 1,000 and 1,500 Hz.
There was no statistically significant difference between pre and post-treatment DPOAE responses and ABR thresholds group 2 and 3. However, the group 1 that administered only cisplatin treatment demonstrated significant deterioration of the ABR thresholds and DPOAE responses, except for 1,000 and 1,500 Hz.
Discussion
The present study showed that treatment with TQ may play a protective role against cisplatin-induced ototoxicity in rats. To our knowledge, there is no study on this issue in the literature. Our results showed that DPOAE responses and ABR thresholds were preserved in the group 2 (cisplatin plus TQ treatment) when compared with group 1 (only cisplatin treatment).
Pathogenesis of medicine-originated ototoxicity may be linked with the accumulation of medicines within cells. Laboratory animals as well as in vitro studies have demonstrated that cisplatin leads to hearing loss by affecting various regions of the cochlea. The most commonly reported histopathological manifestation of ototoxicity is outer hair cells degeneration. At the beginning, outer hair cells stereocilia tip-links are damaged, which is followed by a loss of outer hair cells [4]. Cisplatin ototoxicity progressively damages cochlear outer hair cells, from the base to the apex and damage also includes collapse of Reissner’s membrane, atrophy of the stria vascularis and supporting cells the organ of Corti [16]. In our study in the cisplatin group, DPOAE amplitudes decreased in the higher frequencies but the lower frequencies were spared. This is the good sign indicating the fact that cisplatin progressively destroys base of the cochlea first and travels towards the apex. We demonstrated that use of cisplatin in rats caused elevation of ABR thresholds and decrease of DPOAE responses, which suggested ototoxicity. Moreever, this ototoxic effect may be deep due to accumulation of cisplatin in inner ear. But, we performed only one control measurement 3 days after drug administration. In fact, this is a limitation of our study because we did not perform repetitive measurements later.
The exact cellular and molecular mechanisms of cisplatin-induced ototoxicity are still not well known. But, recent studies have proposed that one of the underlying mechanisms of the pathogenesis of ototoxicity may be oxidative stress. There are studies linking cisplatin-induced ototoxicity to excessive production of free oxygen radicals in the cochlea, outer hair cells, spiral ligament, stria vascularis and spiral ganglionic cells [1]. Cisplatin also decreases antioxidant enzymes that would scavenge and neutralize increased superoxidase [1]. Cisplatin accumulates in the cochlear tissues, integrates into the DNA and causes dysfunctional proteins and enzyme synthesis. This leads to generate excessive free oxygen radicals combined with decreased antioxidant enzyme system. The biologic effects of free oxygen radicals are controlled in vivo by a wide spectrum of enzymatic and non-enzymatic defense mechanisms Extreme increment in the production of free oxygen radicals or decrement in the antioxidant system results in potentially cytotoxic oxidative stress [23]. Once the stability between free oxygen radicals production and the antioxidative defiance mechanisms is impaired, oxidative stress can occur, which can result in cochlear cell injury or death.
Endogenous or exogenous antioxidant agents may protect against cisplatin-induced ototoxicity. Exogenous administration of antioxidant agents has been used to reduce cisplatin ototoxicity in experimental animal studies, most likely by scavenging free oxygen radicals. These have included resveratrol, melatonin, dexamethasone, Vitamine E, d-methionine, N-acetylcysteine, gingko biloba extract, ebselen, amiphostine, sodium salicylate, Salvia miltiorrhiza and pomegranate extract [4–16]. Up to know, there is no FDA-approved agent that has shown efficacy in preventing cisplatin ototoxicity [4].
TQ has been the focal point in the pharmacological studies in recent years due to its strong antioxidant property. However, the exact molecular mechanism of TQ protective function connected with antioxidant activity is not well understood. Studies with laboratory animals as well as in vitro studies demonstrate that TQ can function as a scavenger of different free oxygen radicals including superoxide radical anion and hydroxyl radicals [24, 25]. It also increases the level of antioxidant enzymes such as glutathione peroxidase, glutathione reductase and catalase [18]. In addition, previous experimental animal studies demonstrated that TQ might have a protective effect for several organs against cardiotoxicity, hepatotoxicity and nephrotoxicity induced by various agents. These agents include the doxorubicine-induced cardiotoxicity, carbon tetrachloride-induced hepatotoxicity, vancomycin and cisplatin-induced nephrotoxicity [18, 20–22]. To our knowledge, the protective effect of TQ in cisplatin ototoxicity has not been previously reported. This present study showed that in the cisplatin plus TQ-treated group, DPOAE responses and ABR thresholds were preserved when compared with the group receiving cisplatin alone.
There may be a problem about reducing antitumor activity of cisplatin because of TQ administration. Cisplatin antitumor activity is associated with the inhibition of the DNA synthesis. Cisplatin antitumor activity results from intracellular binding of an activated, positively charged form with a nucleophilic site on DNA to form bifunctional covalent links that interfere with normal DNA function [4, 16]. TQ has demonstrated its therapeutic effects in many different types of malignancies [26]. The antitumor effects of TQ are mediated through different modes of action, including anti-proliferation, cell cycle arrest, apoptosis induction, suppression of cancer metastasis and angiogenesis, reactive oxygen species generation and synergism with conventional medicine [27]. The antitumor activity of TQ has been investigated in tumor xenograft mice models for pancreatic, prostate, colon and lung cancer [28–31]. These studies suggest that TQ not only exerts potent anti-tumor effect, but can also potentate the therapeutic efficacy of commonly employed chemotherapeutic drugs for cancer treatment. Combination of TQ and conventional chemotherapeutic drugs could result in greater antitumor effect. Jafri et al. [31] demonstrated that greater anti-tumor effect of TQ in combination with cisplatin on NCI-H460 non-small cell lung cancer cells. This encouraged us to study with TQ. Although in previous studies antitumor activity of TQ has been shown, future studies are need to evaluate TQ’s affect on cisplatin’s antitumor activity.
As a matter of fact, there are some limitations of our study that we have to mention. First, we did not measure levels of oxidative stress markers and antioxidants before and at the end of drug administration. Therefore, TQ protective function may due to its anti-inflammatory, immunomodulatory and antioxidant activity together on cisplatin-induced ototoxicity. Second, we did not investigate comparison with TQ and other protective agents against cisplatin-induced ototoxicity. While demonstration, protective effect of a new agent also needs comparison with already known agents in relation to effectiveness and costs. Third, we performed only one control measurement 3 days after cisplatin administration and we did not perform repetitive measurements later. Its ototoxic effect may be deep due to accumulation of cisplatin in inner ear later. Fourth, the small size of animal sample represents another important limitation. However, we performed a post hoc power analysis based on post-treatment ABR results (effect size: 1, α: 0.05) that revealed the study power as 99 %. Finally, this is a preliminary study; however, we conclude that cisplatin-induced ototoxicity may be prevented by TQ use in the rats.
Conclusion
This is the first study to investigate the protective effects of TQ against cisplatin-induced ototoxicity. Our results suggest that DPOAE responses and ABR thresholds were preserved in the cisplatin plus TQ-treated group when compared with the group receiving cisplatin alone. In light of these findings, we conclude that cisplatin-induced ototoxicity may be prevented by TQ use in the rats. However, further studies which have combined with more definitive electrophysiological and histopathological examinations are needed to evaluate the protective effect of the TQ on cisplatin-induced ototoxicity.
References
Rybak LP, Mukherjea D, Jajoo S, Ramkumar V (2009) Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med 219:177–186
Williams CJ, Whitehouse JM (1979) Cis-platinum: a new anticancer agent. Br Med J 1:1689–1691
Hartmann JT, Lipp HP (2003) Toxicity of platinum compounds. Expert Opin Pharmacother 4:889–901
Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, Sirvanci S, Yuksel M, Uneri C, Tutkun A (2012) Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol 76:404–408
Erdem T, Bayindir T, Filiz A, Iraz M, Selimoglu E (2011) The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Otorhinolaryngol 269:2185–2188
Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F (2000) Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res 28:73–80
Daldal A, Odabasi O, Serbetcioglu B (2007) The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg 137:747–752
Kalkanis JG, Whitworth C, Rybak LP (2004) Vitamin E reduces cisplatin ototoxicity. Laryngoscope 114:538–542
Campbell KC, Meech RP, Rybak LP, Hughes LF (1999) d-Methionine protects against cisplatin damage to the stria vascularis. Hear Res 138:13–28
Feghali JG, Liu W, Van De Water TR (2001) L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 111:1147–1155
Huang X, Whitworth CA, Rybak LP (2007) Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol 28:828–833
Lynch ED, Gu R, Pierce C, Kil J (2005) Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res 201:81–89
Church MW, Blakley BW, Burgio DL, Gupta AK (2004) WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol 5:227–237
Hyppolito MA, de Oliveira JA, Rossato M (2006) Cisplatin ototoxicity and otoprotection with sodium salicylate. Eur Arch Otorhinolaryngol 263:798–803
Xu O, Liu Y, Li X, Yang Y, Zhang Z, Wang N, Zhang Y, Lu H (2011) Protective effects of Salvia miltiorrhiza against cisplatin-induced ototoxicity in guinea pigs. Am J Otolaryngol 32:228–234
Yazici ZM, Meric A, Midi A, Arınc YV, Kahya V, Hafız G (2012) Reduction of cisplatin ototoxicity in rats by oral administration of pomegranate extract. Eur Arch Otorhinolaryngol 269:45–52
Gökçe A, Oktar S, Koc A, Yonden Z (2011) Protective effects of thymoquinone against methotrexate-induced testicular injury. Hum Exp Toxicol 30:897–903
Basarslan F, Yilmaz N, Ates S, Ozgur T, Tutanc M, Motor VK, Arica V, Yilmaz C, Inci M, Buyukbas S (2012) Protective effects of thymoquinone on vancomycin-induced nephrotoxicity in rats. Hum Exp Toxicol 16:E167–E171
Nader MA, el-Agamy DS, Suddek GM (2010) Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res 33:637–643
Badary OA, Nagi MN, Al-Shabanah OA, Al-Sawaf HA, Al-Sohaibani MO, Al-Bekairi AM (1997) Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol 75:1356–1361
Nagi MN, Mansour MA (2000) Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res 41:283–289
Nagi MN, Alam K, Badary OA, Al-Shabanah OA, Al-Sawaf HA, Al-Bekairi AM (1999) Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. Biochem Mol Biol Int 47:153–159
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721–724
Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM (2002) Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct 20:143–151
Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH et al (2003) Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 26:87–98
Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, Sarkar FH, Mohammad RM (2010) Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer 62:938–946
Woo CC, Kumar AP, Sethi G, Tan KH (2012) Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol 83:443–451
Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM (2009) Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res 69:5575–5583
Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M, Dou QP, Reddy GP (2007) Androgen receptor and E2F–1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res 67:7782–7788
Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B, Jurjus A, Roessner A, Schneider-Stock R (2008) Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med 12:330–342
Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H (2010) Thymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J Exp Clin Cancer Res 29:87
Conflict of interest
None of the authors have a financial relationship with any organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sagit, M., Korkmaz, F., Akcadag, A. et al. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol 270, 2231–2237 (2013). https://doi.org/10.1007/s00405-012-2254-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-2254-6