Abstract
Secondary and tertiary hyperparathyroidism (HPT) develop in patients with renal failure due to a variety of mechanisms including increased phosphorus and fibroblast growth factor 23 (FGF23), and decreased calcium and 1,25-dihydroxy vitamin D levels. Patients present with various bone disorders, cardiovascular disease, and typical laboratory abnormalities. Medical treatment consists of controlling hyperphosphatemia, vitamin D/analog and calcium administration, and calcimimetic agents. Improved medical therapies have led to a decrease in the use of parathyroidectomy (PTX). The surgical indications include parathyroid hormone (PTH) levels >800 pg/ml associated with hypercalcemia and/or hyperphosphatemia despite medical therapy. Other indications include calciphylaxis, fractures, bone pain or pruritis. Transplant recipients often show decreased PTH, calcium and phosphorus levels, but some will have persistent HPT. Evidence suggests that PTX may cause deterioration in renal graft function in the short-term calling into the question the indications for PTX in these patients. Pre-operative imaging is only occasionally helpful except in re-operative PTX. Operative approaches include subtotal PTX, total PTX with or without autotransplantation, and possible thymectomy. Each approach has its proponents, advantages and disadvantages which are discussed. Intraoperative PTH monitoring has a high positive predictive value of cure but a poor negative predictive value and therefore is of limited utility. Hypocalcemia is the most common complication requiring aggressive calcium administration. Benefits of surgery may include improved survival, bone mineral density and alleviation of symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background—pathophysiology
The pathogenesis of secondary hyperparathyroidism (2HPT) is complex and incompletely understood, commonly arising in the setting of chronic kidney disease (CKD), first described by Albright et al. in 1934 [1]. The pathogenesis is complex and remains somewhat enigmatic. Impaired urinary phosphate excretion and hyperphosphatemia, along with decreased activity of renal 1-alpha hydroxylase, which converts 25-hydroxy vitamin D (25 OHD) to 1,25-dihydroxy vitamin D (1,25 (OH)2D), have been implicated in the functional reduction of nephrons in CKD. Both result in hypocalcemia, leading to 2HPT. However, recent data have indicated the important role of the phosphotonin FGF23 in CKD and subsequently 2HPT. FGF23 has been found to be elevated in early CKD, prior to elevations of phosphate and parathyroid hormone (PTH) [2, 3]. FGF23 decreases phosphate reabsorption in the proximal tubules and reduces 1,25(OH)2D levels through decreased expression of 1-alpha hydroxylase and 24-hydroxylase [3]. FGF23 also has an inhibitory effect on PTH secretion, though given the potential for extremely high levels of PTH in end-stage renal disease (ESRD), the importance of this effect is not clear. High circulating FGF23 is associated with worse patient outcome and survival, as well as diminished PTH response to vitamin D or vitamin D analog treatment. 2HPT in kidney disease therefore develops due to a combination of factors, including hyperphosphatemia, elevated FGF23, reduced 1,25 (OH)2D and 25 OHD, hypocalcemia, and decreased PTH clearance. As many as 90% of CKD patients will develop 2HPT by the time they reach ESRD [4].
Continuous overstimulation of the parathyroid glands due to hyperphosphatemia, low 1,25 (OH)2D and intermittent hypocalcemia leads to diffuse hyperplasia [5]. Over time, changes in the expression of calcium and vitamin D receptors promote nodular hyperplasia and potentiate adenomatous changes [6–9]. The parathyroid glands may then become autonomous and unresponsive to negative feedback mechanisms such as elevated calcium, and administration of calcitriol. Tertiary hyperparathyroidism (3HPT) is autonomous disease resulting from irreversible enlargement and nodularity of the parathyroid glands. It usually occurs in a setting of renal insufficiency, generally manifesting in low to mid normal serum calcium levels, and often with associated significant hypercalciuria [10]. 3HPT generally arises in a subset of patients with 2HPT often manifesting after renal transplantation. It may only be recognizable after transplantation when the underlying renal disease has been cured, but hyperparathyroidism (HPT) persists.
Clinical presentation
Early development of elevated PTH levels is common in CKD with mineral bone disorder. Subsequently, hypocalcemia and hyperphosphatemia are seen, followed by manifestations of bone and vascular disease [11].
The bone disorders which occur in CKD with disordered mineral metabolism are referred to collectively as renal osteodystrophy. This term refers to abnormalities in bone turnover, mineralization and volume, which are quantifiable by bone biopsy [10]. Bone turnover is classically divided into either low (adynamic bone disease), high (osteitis fibrosa cystica), or a combination of both a high and low state (mixed uremic osteodystrophy). A high turnover state, characterized by increased bone formation and resorption, is seen in HPT. Some patients will have evidence of more than one type. Historically, complications of renal osteodystrophy such as fractures, bone pain, and deformity were common. However, today with improved and earlier use of medical therapy most patients remain free of symptoms.
2HPT has been linked to higher cardiovascular morbidity and mortality in patients with kidney disease [12–17]. It is difficult to link this to 2HPT alone, as hyperphosphatemia and an elevated calcium-phosphorus product, commonly present in patients with CKD and 2HPT, have also been identified as independent risk factors for higher cardiovascular mortality and more severe progression of renal disease [18–20]. It has been postulated that the derangements in calcium and phosphate that result from HPT accelerate vascular calcification, including coronary artery calcification, and thereby increase adverse cardiovascular events. Recent evidence suggests that FGF23 concentrations increase in early stages of CKD probably in response to the increasing phosphate. In some experimental models, FGF23 can induce changes in arterial smooth muscle myocytes to induce osteoblast-like cell formation and this may be an important step in the production of vascular calcification in CKD patients. Early control of phosphate concentrations using phosphate binders and prevention of increases in FGF23 may be a therapeutic approach in the future [21–23].
Calciphylaxis, the calcification of small arterial vessels which results in ischemic skin necrosis, and uremic pruritus, a severe itching experienced by a subset of patients on hemodialysis, are thought to be due to changes in calcium and phosphate metabolism.
Medical treatment
Medical management of renal HPT currently focuses on decreasing stimulation of the parathyroid gland with both preventative and therapeutic measures. Because of the interdependence of calcium, phosphate, and vitamin D metabolism, multiple pathways can be targeted by medical therapies centered around three main principles:
-
Controlling hyperphosphatemia (dietary phosphate restriction, phosphate binders)
-
Vitamin D/analog replacement
-
Calcimimetic agents and calcium supplementation
Phosphate control is achieved through dietary restriction and oral phosphate-binding agents. The major modalities available are calcium salts and the non-calcium containing phosphate binders sevelamer (Renagel) and lanthanum carbonate (Fosrenol). Although there is some suggestion that calcium salts may increase the risk of vascular calcification, there are no clinical studies that compare these agents in terms of mortality outcomes. There are no prospective studies which identify a target value for phosphate, but high and uncontrolled phosphate levels are associated with increased mortality [24].
There is evidence that giving Vitamin D2 (ergocalciferol) or D3 (cholecalciferol) for 25 OHD deficiency, defined as <30 ng/ml (74.9 nmol/l), in early stage CKD can lower PTH [25]. Once PTH levels remain persistently above goal (>300 pg/ml for patients on dialysis), active Vitamin D analogs (e.g. calcitriol) should be started. While effective at decreasing serum PTH, Vitamin D analogs may also cause increases in calcium and phosphate. Newer analogs, such as paricalcitol and doxercalciferol, have been developed and have lower toxicity profiles [26].
Calcimimetic agents have transformed the medical treatment of 2HPT. They function by increasing the sensitivity of the calcium-sensing receptor (CaSR) in the parathyroid gland. Currently cinacalcet (Sensipar) is the only available oral calcimimetic, and has been studied extensively. The largest trial consisted of 1,136 hemodialysis patients and found that cinacalcet significantly increased the likelihood of achieving goal PTH (≤300 pg/mL), calcium (8.4–9.5 mg/dL), phosphorus (3.5–5.5 mg/dL), and calcium-phosphorus product (<55 mg2/dL2) as well as decreasing the risk of fracture, cardiovascular hospitalization, and the number of patients undergoing parathyroidectomy (PTX) [27, 28].
Surgical indications
Parathyroidectomy for renal HPT is less common since the advent of improved medical therapies, with one retrospective survey of US national data finding a 30% decline in the 1990s [29]. This is likely attributable to improved control of hyperphosphatemia and calcitriol supplementation. An analysis of four randomized controlled trials shows that use of cinacalcet is associated with decreased need for PTX [24]. Currently 1–2% of patients with 2HPT will undergo surgery each year, with ~10% eventually undergoing PTX [30, 31].
The indications for PTX are not well established. Traditionally, surgical therapy has been offered to patients who have persistently elevated PTH with elevated calcium and/or phosphate despite maximal medical therapy, and for those who are symptomatic (fractures, bone pain, pruritus) or experience adverse side effects from the medications. Guidance for selecting patients for PTX is significantly limited by the lack of randomized controlled trials comparing medical and surgical therapies. The National Kidney Foundation’s Kidney Disease Quality Outcomes Initiative (KDOQI) recently published guidelines for PTX in patients with CKD. The KDOQI guidelines set forth the opinion that patients with severe HPT (PTH >800 pg/mL) associated with hypercalcemia and/or hyperphosphatemia, despite medical therapy, should be offered PTX. There are no studies showing biochemical marker thresholds at which point PTX is beneficial, but most patients undergoing PTX for renal HPT have PTH levels >800 pg/mL, therefore this number is often cited as a threshold for offering surgery [32] (see Table 1). One consideration is that the eradication of hypercalcemia due to HPT allows the important therapy of exogenous vitamin D therapy to resume.
Calciphylaxis is considered a strong indicator for PTX. In calciphylaxis, high levels of phosphate bind with calcium and contribute to intravascular and soft tissue calcification. This presents as purpuric, painful cutaneous lesions that progress to dry gangrene if untreated. Calcium may be normal or slightly elevated. Mortality rates associated with calciphylaxis may be as high as 90%, according to retrospective case series [33, 34]. Women are three times as likely to suffer from calciphylaxis [34, 35]. Diagnosis is based on classic skin findings and may be confirmed by biopsy. Biopsy, however, is considered controversial by some as the lesions are characterized by poor healing. Girotto et al. [36] conducted a retrospective review of 13 patients with calciphylaxis and found a significant increase in survival for patients treated with PTX, with median survival 36 months, versus 3 months without surgery (p = 0.021). Another series of 16 patients found increased survival with operation (14.8 vs. 6.3 months), however, this finding did not reach statistical significance [34]. Several studies suggest that PTX is also associated with decreased pain and rapid healing of cutaneous wounds [36–38]. Girotto et al. [36] found resolution of pain and healing of cutaneous wounds in all PTX patients with calciphylaxis. Duffy et al. [39] performed a retrospective study of 15 patients with calciphylaxis showing partial or complete wound healing in all PTX patients and a longer median survival in the PTX group (39 vs. 3 months; p = 0.017).
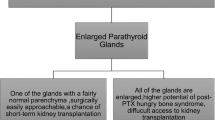
Parathyroid gland size and nodularity have been proposed as an indication for surgical treatment. Tominaga et al. [40] demonstrated that >85% of glands weighing >500 mg contain hyperplastic nodules. Nodular parathyroid tissue has a decreased response to inhibitory stimuli and may be less likely to respond to medical management [41, 42]. However, there have been no studies determining whether or not glands >500 mg are more likely to fail medical management. In renal transplant patients with 3HPT, nodular or adenomatous glands are believed to be less likely to regress, and weight >500 mg as estimated by various imaging techniques is gaining in popularity as a criteria for PTX [31].
Considerations for renal transplant recipients
Restoration of normal renal function after renal transplantation results in significant improvement of HPT with a 63% pre-transplant prevalence declining to 17% at 1 year after transplantation [43, 44]. Rapid decrease in PTH during the first 3–6 months after transplantation is attributed to reduction in functional parathyroid mass, but further gradual decline in PTH has been observed beyond the first year after transplantation [43, 44]. Traditionally, the prevalence of 3HPT has been estimated to be 2–5% of the ESRD population. Recent reports give a much higher prevalence of persistently elevated PTH at 1 year after transplantation (17–30%) [44–47]. While some have ascribed this discrepancy to differing definitions of 3HPT, it is likely that the true prevalence of 3HPT is higher than previously thought. There are genetic differences in susceptibility to HPT, as conferred by vitamin D receptor polymorphism, possibly explaining some of the variance in prevalence of 3HPT among different study groups [48]. Regardless of genetic differences, patients with 3HPT who do not normalize PTH are more likely to have been on dialysis longer and have significantly higher pre-transplant PTH levels than those who normalize PTH post-transplantation [44, 48, 49].
Renal transplant recipients with 3HPT who are symptomatic or have persistent hypercalcemia may be considered candidates for PTX (Table 2) [31]. Potential benefits of correcting 3HPT include resolution of symptoms, prevention of fractures, prevention of nephrocalcinosis, as well as possible improvement of hypertension and dyslipidemia [50]. Traditionally, it has been recommended that PTX be delayed for 12 months after renal transplantation. Advocates of a shorter delay of 3 months cite studies showing that the majority of post-transplant decrease in PTH occurs during the first 3 months [44, 48]. Currently, there is no published study comparing the outcomes of PTX after 3-month delay versus 12-month delay.
It is well known that PTX after renal transplantation is associated with decreased renal graft function, as evidenced by increasing serum creatinine. This decline in graft function is observed immediately after PTX and can persist beyond 12 months [50–52]. Return of serum creatinine to baseline can be as rapid as 3 months after PTX but may be prolonged in those patients with elevated baseline creatinine (Cr >2.0 mg/dL) and higher pre-PTX PTH [50–54]. This decline in graft function has been attributed to decreased PTH, which has a vasodilatory effect on preglomerular renal arteries [52, 55]. A similar decline in renal function has also been observed in pre-dialysis CKD patients undergoing PTX, and oliguric ESRD patients on hemodialysis have become anuric after PTX [56, 57]. A greater decrease in PTH after PTX among transplant recipients has been associated with prolonged decreased graft function [54].
Given these findings, there has been much debate about the appropriateness of PTX for renal transplant recipients. Because most patients with 3HPT present with diffuse parathyroid hyperplasia, the traditionally accepted surgical techniques have been total parathyroidectomy (TPTX) with or without autotransplantation or subtotal parathyroidectomy (SPTX) [31, 58]. Schlosser et al. [52] found that 18 patients undergoing total PTX with autotransplantation in the setting of pre-existing graft dysfunction (pre-PTX Cr >2.0 mg/dL) had significant decline in renal graft function after PTX, persisting for up to 1 year. Seventeen patients undergoing total PTX with autotransplantation in the setting of pre-PTX Cr <2.0 mg/dL had smaller (but statistically significant) declines in renal graft function, whereas 12 patients undergoing less than all 4 gland removal (including subtotal PTX) did not experience a decline in renal graft function. Other studies reporting on post-PTX renal graft function have not differentiated between types of PTX and those studies reporting on different types of PTX have not reported post-PTX renal graft function. Theoretically, there may be an advantage to performing subtotal PTX over total PTX, given the association of greater decrease in PTH after total PTX with prolonged post-PTX renal graft dysfunction. More research is needed to determine if there is any substantive and lasting advantage to performing subtotal PTX in renal transplant recipients.
Subgroups of renal transplant recipients with HPT may have one or more parathyroid adenomas rather than four gland hyperplasia. Pitt et al. [59] from the University of Wisconsin have advocated limited PTX in this minority of patients if up to two adenomas are found after four gland identification. They did not routinely biopsy the normal appearing glands but performed intraoperative PTH (IOPTH) monitoring to verify normalization of PTH. Twenty-nine patients were treated in this manner without an increased rate of recurrence as measured by calcium and PTH 6 months after PTX with a mean follow-up of 78 months [47, 59]. Others have found an increased risk of recurrence when less than subtotal PTX was performed, but these studies have inadequate power to make any conclusive recommendation (n = 11 Triponez vs. n = 29 Pitt) [59, 60].
Despite decreased graft function after PTX, there are no firm data to suggest that PTX is ultimately harmful for transplant graft survival. Lee et al. [61] found decreased graft survival after PTX among 22 patients, but overall graft survival was only 10% at 6 years, suggesting confounding factors. Three other studies (n = 185 in total) did not find a statistically significant effect of PTX on graft survival despite decrease in graft function [51, 54, 60]. Further studies with greater sample size, longer follow-up, and differentiation between types of operation are needed to clarify the effects of PTX on renal transplant graft survival.
Pre-operative imaging
Though considered standard by most endocrine surgeons in primary HPT, pre-operative imaging has not been routinely done for 2HPT or 3HPT. In theory, pre-operative imaging might help to locate an ectopic gland, or help to localize a single lesion in a re-operative case. However, the sensitivities of pre-operative imaging modalities, including 99mTc-sestamibi and ultrasound, are poor in multigland disease and in renal HPT [62, 63]. Milas et al. [64] investigated pre-operative localization in 2HPT or 3HPT, and found that pre-operative 99mTc-sestamibi scanning failed to accurately localize all parathyroid glands in any patient, and localized mediastinal parathyroid glands in only three of eight patients with mediastinal lesions identified at time of surgery. Pre-operative imaging did not locate any ectopic or supernumerary glands in any of the 30% of the patients who had one [65]. A recent study by Wimmer et al. [66] found that computed tomography-(99m)Tc-sestamibi-single photon emission computed tomography (CT-MIBI-SPECT) image fusion identified abnormal glands in 46.7% (14/30) of the patients with multiglandular disease of any type. CT scan alone found all glands in 36.7% (11/30) and MIBI-SPECT alone in 13.3% (4/30) of patients. These studies suggest that routine pre-operative localization studies before PTX in 2HPT or 3HPT are not as helpful as they would seem to be theoretically. We do not advise against performing the studies, as they are non-invasive and may occasionally be helpful, but they do increase the cost and duration of treatment. Extensive attempts at localization using multiple imaging techniques are not indicated prior to initial exploration.
On the other hand, in the event of persistent or recurrent HPT after PTX, localization imaging is considered standard because usually only a single abnormal gland remains. Ultrasound, 99mTc-sestamibi, CT and MRI are the most commonly used modalities. Combination modalities such as CT-MIBI-SPECT are also gaining in popularity [67]. Fusion imaging combining MIBI-SPECT with contrast enhanced CT has been employed by one of the authors (PKP) with a positive predictive value of 91%, although the majority of patients in this series had primary HPT [68]. This modality exploits both imaging techniques in providing an anatomic/physiologic localization corollary which expedites re-exploration in a surgical bed usually compromised by postoperative fibrosis. In patients with persistent or recurrent 2HPT, 99mTc-sestamibi has been shown to successfully identify the parathyroid lesion in 85% of the patients [69]. Nodules found in the neck may be aspirated and the aspirate sent for PTH measurement to verify the diagnosis, as a supplement to microscopic examination. Sampling of the venous drainage proximal to an autograft with PTH measurement can help determine if recurrent HPT is secondary to forearm graft hyperplasia [31]. Selective venous sampling of neck and chest veins may be useful in cases where other imaging is unrevealing.
Operative treatment
Operative approach
There are three main surgical approaches to PTX in patients with 2HPT or 3HPT. The primary surgical procedures are:
-
SPTX
-
TPTX with autotransplantation
-
TPTX without autotransplantation
Subtotal parathyroidectomy is the resection of 3½ parathyroid glands, leaving a remnant of a parathyroid gland in situ. A bilateral neck exploration is done, and the most normal appearing parathyroid gland is identified. A vascularized part of this gland is marked with a metallic clip and left in situ. Prior to resecting the other glands, the preserved remnant must be examined for viability. It is preferable to save an inferior gland as they are usually located more anteriorly in the neck, making the remnant more accessible to reoperation if necessary. The histological nodularity of the glands must be noted as nodular hyperplasia is associated with increased rates of postoperative hypertrophy [40]. Exploration and resection of any observed cervical thymic tissue may be required in an effort to remove any supernumerary parathyroid glands or parathyroid rests—defined as histologically identifiable parathyroid tissue not comprising and distinct from the parathyroid glands—from within the visualized thymus (see “Thymectomy” below).
Total parathyroidectomy with or without autotransplantation involves careful identification of all four parathyroid and any supernumerary glands. For autotransplantation, the most normal appearing gland is identified and then a portion of it is minced into 1–2 mm pieces for re-implantation. The sternocleidomastoid muscle, the brachioradialis muscle, or the subcutaneous fat of the forearm are all potential sites for re-implantation. In all cases, the site of re-implantation should be marked with a metallic clip or non absorbable suture material so it may be identified in the future if necessary [67, 70]. Re-implantation into the forearm may obviate the need for surgical re-exploration of the neck in the event of recurrent HPT due to the graft. Ideally, cryopreservation should be available if TPTX without autotransplantation is the procedure of choice, and in the event that TPTX with autotransplantation is not sufficient for sustaining normal calcium homeostasis. Although rarely required parathyroid tissue can be held in reserve for delayed re-implantation. The optimal site for re-implantation of parathyroid glands in patients with 3HPT is the forearm. A majority of these patients have an arteriovenous fistula from their dialysis, so the graft should be placed into the non-fistula arm, if available. A major advantage of implanting the parathyroid gland in the forearm is that blood can be drawn from both arms (downstream from the graft) to confirm the diagnosis of graft-related recurrence, without the need for further localization studies. No large randomized controlled trials comparing one surgical approach to another exist, and choice of procedure is largely left to surgeon and nephrologist preference. Those who favor SPTX point to studies that suggest decreased rates of hypoparathyroidism with comparable recurrent HPT rates when compared to TPTX with autotransplantation [71, 72]. Melck et al. [73] have suggested that forearm debulking for recurrences following TPTX may be more difficult as a result of forearm parathyromatosis (seen in 4/7 cases) necessitating en block resection. Advocates of TPTX with autotransplantation quote studies that suggest a decreased incidence of HPT recurrence. In 1991, Rothmund et al. [74] randomized 40 patients to either SPTX or TPTX with autotransplantation. Their findings suggested significantly decreased rates of recurrence with TPTX and autotransplantation, as well as normalization of serum calcium and phosphorus. No patient required medical or surgical intervention for hyperfunction of the remaining parathyroid tissue [74]. However, this single randomized trial was too small to lead to standardization of operative approach. A meta-analysis of 53 publications on operation for 2HPT found that re-operative findings of supernumerary glands, missed in situ glands, and negative re-explorations occurred at equal rates for both operations [75]. Advocates of TPTX without autotransplantation point to lower rates of recurrence (0–4%) compared with STPX or TPTX with autotransplantation (5–80%). Rates of persistent hypoparathyroidism after TPTX without autotransplantation have been reported as low as 0–7% [76–79]. A recent large retrospective study by Coulston et al. [80], however, found a 12.2% (14/115) recurrence rate in patients undergoing TPTX without autotransplantation. There is not enough evidence-based information available at this time to declare one approach superior to another.
Subtotal parathyroidectomy is usually the preferred operation for 3HPT after renal transplant. While there are no randomized controlled trials showing superiority over TPTX with autotransplantation for 3HPT, retrospective studies suggest outcomes are similar for the two approaches [71, 81, 82]. Other studies suggest the risk of permanent hypoparathyroidism is less likely with SPTX. In addition, Triponez et al. [83] conducted a retrospective analysis of 74 patients with 3HPT, showing a 5.2 times increased risk of persistent or recurrent HPT with less than SPTX compared to SPTX. Although there are no data to show improved long-term renal graft survival, there are data to suggest at least improved short-term renal graft function with SPTX as compared to TPTX (see above section on considerations for renal transplant recipients) [51, 54, 60, 61, 84]. Others advocate the even more selective approach of limited or focused PTX. In some series, as many as 30% of the patients with 3HPT will have one or two adenomas, and studies are reporting high success rates in terms of symptom relief and normalization of calcium with resection of the most abnormal appearing glands with use of pre-operative localization and IOPTH [68, 85, 86]. Larger studies with longer follow-up are required to further investigate the efficacy of such limited resections.
Thymectomy
Cervical thymectomy is considered by many to be an important component of any operative treatment for 2HPT or 3HPT. Autopsy series suggest the prevalence of supernumerary parathyroid glands is 13% in the general population [87]. However, surgical series suggest the prevalence may be as high as 30%, many of which are located in thymic tissue [82, 88–90]. Parathyroid rests can be found in extraparathyroidal tissue, most commonly the thymus, in up to 37% of cases. The ongoing stimulus of renal failure contributes to the hyperplasia of these parathyroid rests and is a potential source for recurrent HPT after PTX [89, 91]. While some advocate thymectomy for all patients, others limit thymectomy to cases in which four glands are not identified [31].
Intraoperative PTH monitoring
Parathyroid hormone—an 84 amino acid polypeptide—and its fragments are eliminated by the liver and kidneys. In renal failure, clearance of PTH and its fragments is impaired. First generation PTH assays measured only one segment of the PTH molecule and were therefore inaccurate. Second generation assays measure intact PTH using two antibodies directed at the N- and C-terminal regions. However, these assays may also overestimate the actual level of PTH because of cross-reactivity with PTH fragments containing amino acids 7–84 which are present in renal failure and have similar functional capacity to 1–84 PTH. A third generation of assays was developed to address this problem which uses antibodies directed to the first 4–6 amino acids of the N-terminal portion which are absent in the 7–84 fragments. It should be noted that the majority of PTH assays used in the rapid intraoperative environment are of the second generation intact PTH variety.
Intraoperative PTH monitoring is becoming standard in primary HPT, but its role in the treatment of 2HPT and 3HPT remains uncertain. The application of IOPTH assessment for renal-induced HPT is less straightforward than that for primary HPT due to the following three factors: (1) The vast majority of renal HPT is caused by the presence of parathyroid hyperplasia and thus at least four abnormal glands must be removed before the PTH level will begin to fall, (2) metabolic changes inherent in renal failure result in a variable rate of clearance of PTH after excision of the hyperfunctioning tissue, and (3) cross reactivity of the intact PTH assay with PTH fragments occurs in renal failure patients [89]. Intact PTH assays may overestimate PTH 1–84 levels due to recognition of non-PTH 1–84 fragments that can accumulate in diminished renal function particularly PTH 7–84 [92, 93].
Data on the accuracy of IOPTH in 2HPT and 3HPT are conflicting [83, 94–98]. Haustein et al. [95] found IOPTH to be helpful and altered surgical management in 16% of the patients, leading to additional parathyroid tissue resection and cure. Bieglmayer et al. [99] demonstrated that in patients with 3HPT and restored renal function following successful transplantation, second generation intact PTH assays accurately predicted success of PTX.
Kaczirek et al. [97] demonstrated the limited value of the IOPTH assay (2nd generation), and the possible superiority of a whole PTH 1–84 assay (3rd generation) in patients undergoing PTX for 2HPT. He concluded that IOPTH testing in RHPT is dependent on both renal function and type of assay [94, 97, 99]. The development of third generation whole PTH assays which measure PTH 1–84 without significant cross reactivity to the accumulated PTH 7–84 fragments in renal failure may improve the ability to predict successful resection of hyperfunctioning parathyroid tissue [95].
Unlike the experience of IOPTH in patients with primary HPT, the kinetic profile of PTH degradation in patients with renal failure appears less straightforward, reflecting the multifactorial influences exerted on PTH metabolism by renal disease. IOPTH has a high positive predictive value of cure, but a poor negative predictive value in patients with renal HPT [58, 97]. Eventually, establishing a predictive capability to assure completeness of resection might guide the surgeon regarding the extent of surgery and the decision of whether to perform a thymectomy. However, at this time the role of IOPTH in 2HPT and 3HPT remains uncertain. We do not recommend against its use, but do advise against unduly prolonging the operation while awaiting reports from multiple blood specimens.
Pathologic considerations
Similar to other parathyroid disease states, the main goal of gross and histologic examination in the intraoperative setting is confirmation of the presence of parathyroid tissue, with the secondary objective being documentation of the size and cellularity as indicators of hyperfunctioning status for a particular gland. Overall, frozen section evaluation has an accuracy of over 99% in confirmation of parathyroid tissue, and can thus be used in conjunction with IOPTH to guide surgical exploration [100]. While intraoperative cytology has been proposed as a quicker alternative to frozen section, its accuracy varies greatly ranging from <70 to 99% and depends on the expertise of the cytopahtologist [92, 93, 101].
The size and configuration of parathyroid glands in 2HPT varies with extent of disease; earlier states are generally reflected by a diffuse hyperplasia while later stages are characterized by nodular and more asymmetric hyperplasia. In general, resected parathyroid specimens are clearly enlarged and often well above 500 mg ranging from 120 to 6,000 mg in one series of 200 patients [102]. Glands in 3HPT are similarly enlarged ranging from 100 to 7,000 mg [103]. Configuration is somewhat heterogeneous with over 40% of 3HPT glands retaining a diffuse appearance. The parathyroid glands in 2HPT and 3HPT have a very similar histologic appearance to hyperfunctioning parathyroid glands in other disease states. They are histologically composed of nests, cords and solid nodules of chief and oxyphil cells with scant stromal adipose tissue. Though chief cells are the most common histologic cell type, oxyphilic change is fairly common in the glands of 2HPT and 3HPT [103, 104]. One feature, however, that is suggestive of renal HPT is the presence of calcifications in the stroma or arterial walls [105].
Parathyroid glands in 2HPT and 3HPT may pose additional diagnostic challenges in the intraoperative setting because they may show significant fibrosis, cytologic atypia and even mitotic activity [106]. While in primary single gland disease, these criteria are worrisome for parathyroid carcinoma, in the setting of 2HPT and 3HPT, these represent a reactive process that often accompanies extensive nodular change [105, 107]. Carcinomas can rarely arise in the setting of 2HPT and 3HPT with a reported incidence of ~0.2% in one series, and more definitive criteria such as extracapsular, vascular, and perineural invasion, or documentation of a metastasis are required to establish this diagnosis [105, 108–110].
Postoperative considerations
Complications
The most frequent complication of PTX is transient hypocalcemia [63, 109]. This is precipitated by increased bone remineralization, or “hungry bone syndrome”, as well as delayed remnant of autograft function [65, 111, 112]. Transient hypocalcemia often requires aggressive treatment with intravenous and oral calcium, injectable and oral vitamin D/analog supplementation, and high calcium dialysate. This invariably leads to an extended hospitalization. Severe or permanent hypocalcemia requiring admission for longer than 1 week or readmission is uncommon (<7%) [64, 65, 111]. Wound complications and laryngeal nerve injury are very rare (<1%), particularly in experienced hands.
Survival
While high PTH levels, hyperphosphatemia, and vascular calcification are known to increase mortality, there are little data on the effect of PTX on long-term mortality. Information from the United States Renal Database System looking at Medicare and Medicaid patients on dialysis showed an increase short-term mortality after PTX (3.1 vs. 1.2% 30-day mortality), but decrease in long-term mortality (median survival 53 vs. 47 months) [113].
Other benefits
Parathyroidectomy improves bone mineral density (BMD) in patients with 2HPT and 3HPT, ranging from 1 to 23% increase in BMD, with most studies citing numbers around 10–12%. Improvement in BMD can be noticed as early as 1 month postoperatively and improved or stabilized over 2–3 years follow-up [64, 114–117]. However, there is little information on its long-term effect on fracture rates. In one retrospective study, PTX decreased the risk for hip fracture in dialysis patients (RR 0.68, 95% CI 0.54–0.86) [118].
Studies suggest that PTX improves blood pressure control in CKD patients. This is hypothesized to be due to normalization of calcium, which modulates cardiac output and peripheral vascular resistance. Elevated PTH also may have a direct effect on vascular endothelium leading to hypertension [119, 120].
Uremic pruritus, severe itching experienced by a subset of patients on hemodialysis, is often relieved by PTX. PTX may also improve the weakness that is commonly felt by dialysis patients. In a case series by Chou et al. [115], 56 patients with renal HPT had muscle strength evaluated before and after PTX. At 3 months, all patients showed an increase in muscle strength and overall activity. PTX may also improve nutritional status, and humoral and cell mediated immunity in patients with renal HPT [121, 122].
Conclusion
Parathyroidectomy for 2HPT and 3HPT in CKD may benefit a subset of patients who do not respond to medical therapy. Controversy remains concerning the indications for surgical PTX and optimal surgical technique. Clinical decision-making is difficult given the lack of large, prospective studies comparing medical and surgical management, or comparing different surgical techniques. In general, patients who are refractory to medical management manifested by symptoms and/or hyperphosphatemia and less commonly hypercalcemia, should be considered for PTX. At present, there is one ongoing randomized controlled trial comparing TPTX with and without thymectomy and autotransplantation [52]. Future advances in medical management will likely lead to continually changing recommendations on PTX in patients with renal HPT.
References
Albright F (1948) A page out of the history of hyperparathyroidism. J Clin Endocrinol Metab 8:637–657
Hasegawa H, Nagano N, Urakawa I et al (2010) Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78:975–980
Hori M, Shimizu Y, Fukumoto S (2011) Minireview: fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology 152:4–10
Memmos DE, Williams GB, Eastwood JB et al (1982) The role of parathyroidectomy in the management of hyperparathyroidism in patients on maintenance haemodialysis and after renal transplantation. Nephron 30:143–148
Fraser WD (2009) Hyperparathyroidism. Lancet 374:145–158
Drueke TB (1998) Primary and secondary uraemic hyperparathyroidism: from initial clinical observations to recent findings. Nephrol Dial Transplant 13:1384–1387
Llach F, Velasquez Forero F (2001) Secondary hyperparathyroidism in chronic renal failure: pathogenic and clinical aspects. Am J Kidney Dis 38(Suppl 5):20–33
Malberti F, Farina M, Imbasciati E (1999) The PTH-calcium curve and the set point of calcium in primary and secondary hyperparathyroidism. Nephrol Dial Transplant 14:2398–2406
Tominaga Y (1999) Mechanism of parathyroid tumourigenesis in uraemia. Nephrol Dial Transplant 14(Suppl 1):63–65
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int (Suppl 113):1–130
Moe S, Drueke T, Cunningham J et al (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 69:1945–1953
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15:2208–2218
De Boer IH, Gorodetskaya I, Young B, Hsu CY, Chertow GM (2002) The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 13:2762–2769
Fellner SK, Lang RM, Neumann A, Bushinsky DA, Borow KM (1991) Parathyroid hormone and myocardial performance in dialysis patients. Am J Kidney Dis 18:320–325
Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK (2001) Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12:2131–2138
Kalantar-Zadeh K, Kuwae N, Regidor DL et al (2006) Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70:771–780
Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K (2008) Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73:1296–1302
Kestenbaum B, Sampson JN, Rudser KD et al (2005) Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16:520–528
Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP (2006) Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1:825–831
Voormolen N, Noordzij M, Grootendorst DC et al (2007) High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22:2909–2916
Isakova T, Wahl P, Vargas GS et al (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378
Kawarazaki H, Shibagaki Y, Fukumoto S et al (2011) The relative role of fibroblast growth factor 23 and parathyroid hormone in predicting future hypophosphatemia and hypercalcemia after living donor kidney transplantation: a 1-year prospective observational study. Nephrol Dial Transplant 26:2691–2695
Nakai K, Komaba H, Fukagawa M (2010) New insights into the role of fibroblast growth factor 23 in chronic kidney disease. J Nephrol 23:619–625
Ritz E (2005) The clinical management of hyperphosphatemia. J Nephrol 18:221–228
Zisman AL, Hristova M, Ho LT, Sprague SM (2007) Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27:36–43
Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D (2003) Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63:1483–1490
Cunningham J, Danese M, Olson K, Klassen P, Chertow GM (2005) Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 68:1793–1800
Moe SM, Chertow GM, Coburn JW et al (2005) Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 67:760–771
Kestenbaum B, Seliger SL, Gillen DL et al (2004) Parathyroidectomy rates among United States dialysis patients: 1990–1999. Kidney Int 65:282–288
de Francisco AL, Fresnedo GF, Rodrigo E, Piñera C, Amado JA, Arias M (2002) Parathyroidectomy in dialysis patients. Kidney Int (Suppl 80):161–166
Triponez F, Clark OH, Vanrenthergem Y, Evenepoel P (2008) Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg 248:18–30
National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42(Suppl 3):1–201
Coates T, Kirkland GS, Dymock RB et al (1998) Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis 32:384–391
Kang AS, McCarthy JT, Rowland C, Farley DR, van Heerden JA (2000) Is calciphylaxis best treated surgically or medically? Surgery 128:967–971 discussion 971–972
Hafner J, Keusch G, Wahl C, Burg G (1998) Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa 27:137–143
Girotto JA, Harmon JW, Ratner LE, Nicol TL, Wong L, Chen H (2001) Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery 130:645–650 discussion 650–651
Duh QY, Lim RC, Clark OH (1991) Calciphylaxis in secondary hyperparathyroidism. Diagnosis and parathyroidectomy. Arch Surg 126:1213–1218 discussion 1218–1219
Kane WJ, Petty PM, Sterioff S, McCarthy JT, Crotty TB (1996) The uremic gangrene syndrome: improved healing in spontaneously forming wounds following subtotal parathyroidectomy. Plast Reconstr Surg 98:671–678
Duffy A, Schurr M, Warner T, Chen H (2006) Long-term outcomes in patients with calciphylaxis from hyperparathyroidism. Ann Surg Oncol 13:96–102
Tominaga Y, Numano M, Tanaka Y, Uchida K, Takagi H (1997) Surgical treatment of renal hyperparathyroidism. Semin Surg Oncol 13:87–96
Gogusev J, Duchambon P, Hory B et al (1997) Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int 51:328–336
Katoh N, Nakayama M, Shigematsu T et al (2000) Presence of sonographically detectable parathyroid glands can predict resistance to oral pulsed-dose calcitriol treatment of secondary hyperparathyroidism. Am J Kidney Dis 35:465–468
Bonarek H, Merville P, Bonarek M et al (1999) Reduced parathyroid functional mass after successful kidney transplantation. Kidney Int 56:642–649
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y (2004) Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 19:1281–1287
Heaf J, Tvedegaard E, Kanstrup IL, Fogh-Andersen N (2000) Bone loss after renal transplantation: role of hyperparathyroidism, acidosis, cyclosporine and systemic disease. Clin Transplant 14:457–463
Kerby JD, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG (1998) Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 227:878–886
Nichol PF, Starling JR, Mack E, Klovning JJ, Becker BN, Chen H (2002) Long-term follow-up of patients with tertiary hyperparathyroidism treated by resection of a single or double adenoma. Ann Surg 235:673–678 discussion 678–680
Messa P, Sindici C, Cannella G et al (1998) Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int 54:1704–1713
Kinnaert P, Nagy N, Decoster-Gervy C, De Pauw L, Salmon I, Vereerstraeten P (2000) Persistent hyperparathyroidism requiring surgical treatment after kidney transplantation. World J Surg 24:1391–1395
Evenepoel P, Claes K, Kuypers D, Maes B, Vanrenterghem Y (2005) Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant 20:1714–1720
Evenepoel P, Claes K, Kuypers DR, Debruyne F, Vanrenterghem Y (2007) Parathyroidectomy after successful kidney transplantation: a single centre study. Nephrol Dial Transplant 22:1730–1737
Schlosser K, Veit JA, Witte S et al (2007) Comparison of total parathyroidectomy without autotransplantation and without thymectomy versus total parathyroidectomy with autotransplantation and with thymectomy for secondary hyperparathyroidism: TOPAR PILOT-Trial. Trials 8:22
Garcia A, Mazuecos A, Garcia T, González P, Ceballos M, Rivero M (2005) Effect of parathyroidectomy on renal graft function. Transplant Proc 37:1459–1461
Schwarz A, Rustien G, Merkel S, Radermacher J, Haller H (2007) Decreased renal transplant function after parathyroidectomy. Nephrol Dial Transplant 22:584–591
Massfelder T, Parekh N, Endlich K, Saussine C, Steinhausen M, Helwig JJ (1996) Effect of intrarenally infused parathyroid hormone-related protein on renal blood flow and glomerular filtration rate in the anaesthetized rat. Br J Pharmacol 118:1995–2000
Chan YL, Posen S, Savdie E, Caterson RJ, Mahony JF (1983) Total parathyroidectomy and renal function in patients with chronic renal failure. Miner Electrolyte Metab 9:57–61
Tzanakis I, Alifieris E, Kagia S et al (2000) Does parathyroidectomy affect residual diuresis in hemodialysis patients? Nephron 86:402–403
Triponez F, Dosseh D, Hazzan M et al (2006) Accuracy of intra-operative PTH measurement during subtotal parathyroidectomy for tertiary hyperparathyroidism after renal transplantation. Langenbecks Arch Surg 391:561–565
Pitt SC, Panneerselvan R, Chen H, Sippel RS (2009) Tertiary hyperparathyroidism: is less than a subtotal resection ever appropriate? A study of long-term outcomes. Surgery 146:1130–1137
Kandil E, Florman S, Alabbas H et al (2010) Exploring the effect of parathyroidectomy for tertiary hyperparathyroidism after kidney transplantation. Am J Med Sci 339:420–424
Lee PP, Schiffmann L, Offermann G, Beige J (2004) Effects of parathyroidectomy on renal allograft survival. Kidney Blood Press Res 27:191–196
Haciyanli M, Lal G, Morita E, Duh QY, Kebebew E, Clark OH (2003) Accuracy of preoperative localization studies and intraoperative parathyroid hormone assay in patients with primary hyperparathyroidism and double adenoma. J Am Coll Surg 197:739–746
Sebag F, Hubbard JG, Maweja S, Misso C, Tardivet L, Henry JF (2003) Negative preoperative localization studies are highly predictive of multiglandular disease in sporadic primary hyperparathyroidism. Surgery 134:1038–1041 discussion 1041–1042
Milas M, Weber CJ (2004) Near-total parathyroidectomy is beneficial for patients with secondary and tertiary hyperparathyroidism. Surgery 136:1252–1260
Kebebew E, Duh QY, Clark OH (2004) Tertiary hyperparathyroidism: histologic patterns of disease and results of parathyroidectomy. Arch Surg 139:974–977
Wimmer G, Profanter C, Kovacs P et al (2010) CT-MIBI-SPECT image fusion predicts multiglandular disease in hyperparathyroidism. Langenbecks Arch Surg 395:73–80
Chou FF, Chan HM, Huang TJ, Lee CH, Hsu KT (1998) Autotransplantation of parathyroid glands into subcutaneous forearm tissue for renal hyperparathyroidism. Surgery 124:1–5
Pellitteri PK (2003) Directed parathyroid exploration: evolution and evaluation of this approach in a single-institution review of 346 patients. Laryngoscope 113:1857–1869
Chou FF, Lee CH, Chen HY, Chen JB, Hsu KT, Sheen-Chen SM (2002) Persistent and recurrent hyperparathyroidism after total parathyroidectomy with autotransplantation. Ann Surg 235:99–104
Monchik JM, Bendinelli C, Passero MA Jr, Roggin KK (1999) Subcutaneous forearm transplantation of autologous parathyroid tissue in patients with renal hyperparathyroidism. Surgery 126:1152–1158 discussion 1158–1159
Gasparri G, Camandona M, Abbona GC et al (2001) Secondary and tertiary hyperparathyroidism: causes of recurrent disease after 446 parathyroidectomies. Ann Surg 233:65–69
Punch JD, Thompson NW, Merion RM (1995) Subtotal parathyroidectomy in dialysis-dependent and post-renal transplant patients. A 25-year single-center experience. Arch Surg 130:538–542 discussion 542–543
Melck AL, Carty SE, Seethala RR et al (2010) Recurrent hyperparathyroidism and forearm parathyromatosis after total parathyroidectomy. Surgery 148:867–873 discussion 873–875
Rothmund M, Wagner PK, Schark C (1991) Subtotal parathyroidectomy versus total parathyroidectomy and autotransplantation in secondary hyperparathyroidism: a randomized trial. World J Surg 15:745–750
Richards ML, Wormuth J, Bingener J, Sirinek K (2006) Parathyroidectomy in secondary hyperparathyroidism: is there an optimal operative management? Surgery 139:174–180
Ockert S, Willeke F, Richter A et al (2002) Total parathyroidectomy without autotransplantation as a standard procedure in the treatment of secondary hyperparathyroidism. Langenbecks Arch Surg 387:204–209
Pitt SC, Sippel RS, Chen H (2009) Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin N Am 89:1227–1239
Skinner KA, Zuckerbraun L (1996) Recurrent secondary hyperparathyroidism. An argument for total parathyroidectomy. Arch Surg 131:724–727
Zou Q, Wang HY, Zhou J et al (2007) Total parathyroidectomy combined with partial auto-transplantation for the treatment of secondary hyperparathyroidism. Chin Med J (Engl) 120:1777–1782
Coulston JE, Egan R, Willis E, Morgan JD (2010) Total parathyroidectomy without autotransplantation for renal hyperparathyroidism. Br J Surg 97:1674–1679
Tominaga Y, Uchida K, Haba T et al (2001) More than 1,000 cases of total parathyroidectomy with forearm autograft for renal hyperparathyroidism. Am J Kidney Dis 38(Suppl 1):168–171
Triponez F, Dosseh D, Hazzan M, Noel C, Vanhille P, Proye CA (2005) Subtotal parathyroidectomy with thymectomy for autonomous hyperparathyroidism after renal transplantation. Br J Surg 92:1282–1287
Triponez F, Kebebew E, Dosseh D et al (2006) Less-than-subtotal parathyroidectomy increases the risk of persistent/recurrent hyperparathyroidism after parathyroidectomy in tertiary hyperparathyroidism after renal transplantation. Surgery 140:990–997 discussion 997–999
Schlosser K, Endres N, Celik I, Fendrich V, Rothmund M, Fernández ED (2007) Surgical treatment of tertiary hyperparathyroidism: the choice of procedure matters! World J Surg 31:1947–1953
Nichol PF, Mack E, Bianco J, Hayman A, Starling JR, Chen H (2003) Radioguided parathyroidectomy in patients with secondary and tertiary hyperparathyroidism. Surgery 134:713–717 discussion 717–719
Thanasoulis L, Bingener J, Sirinek K, Richards M (2007) A successful application of the intraoperative parathyroid hormone assay in tertiary hyperparathyroidism. Am Surg 73:281–283
Akerström G, Malmaeus J, Bergström R (1984) Surgical anatomy of human parathyroid glands. Surgery 95:14–21
Dumasius V, Angelos P (2010) Parathyroid surgery in renal failure patients. Otolaryngol Clin North Am 43:433–440
Numano M, Tominaga Y, Uchida K, Orihara A, Tanaka Y, Takagi H (1998) Surgical significance of supernumerary parathyroid glands in renal hyperparathyroidism. World J Surg 22:1098–1102 discussion 1103
Pattou FN, Pellissier LC, Noel C, Wambergue F, Huglo DG, Proye CA (2000) Supernumerary parathyroid glands: frequency and surgical significance in treatment of renal hyperparathyroidism. World J Surg 24:1330–1334
Aly A, Douglas M (2003) Embryonic parathyroid rests occur commonly and have implications in the management of secondary hyperparathyroidism. ANZ J Surg 73:284–288
Yao DX, Hoda SA, Yin DY et al (2003) Interpretative problems and preparative technique influence reliability of intraoperative parathyroid touch imprints. Arch Pathol Lab Med 127:64–67
Rohaizak M, Munchar MJ, Meah FA, Jasmi AY (2005) Prospective study comparing scrape cytology with frozen section in the intraoperative identification of parathyroid tissue. Asian J Surg 28:82–85
Chou FF, Lee CH, Chen JB, Hsu KT, Sheen-Chen SM (2002) Intraoperative parathyroid hormone measurement in patients with secondary hyperparathyroidism. Arch Surg 137:341–344
Haustein SV, Mack E, Starling JR, Chen H (2005) The role of intraoperative parathyroid hormone testing in patients with tertiary hyperparathyroidism after renal transplantation. Surgery 138:1066–1071
Kaczirek K, Prager G, Riss P et al (2006) Novel parathyroid hormone (1–84) assay as basis for parathyroid hormone monitoring in renal hyperparathyroidism. Arch Surg 141:129–134
Kaczirek K, Riss P, Wunderer G et al (2005) Quick PTH assay cannot predict incomplete parathyroidectomy in patients with renal hyperparathyroidism. Surgery 137:431–435
Lokey J, Pattou F, Mondragon-Sanchez A et al (2000) Intraoperative decay profile of intact (1–84) parathyroid hormone in surgery for renal hyperparathyroidism–a consecutive series of 80 patients. Surgery 128:1029–1034
Bieglmayer C, Kaczirek K, Prager G, Niederle B (2006) Parathyroid hormone monitoring during total parathyroidectomy for renal hyperparathyroidism: pilot study of the impact of renal function and assay specificity. Clin Chem 52:1112–1119
Westra WH, Pritchett DD, Udelsman R (1998) Intraoperative confirmation of parathyroid tissue during parathyroid exploration: a retrospective evaluation of the frozen section. Am J Surg Pathol 22:538–544
Shidham VB, Asma Z, Rao RN et al (2002) Intraoperative cytology increases the diagnostic accuracy of frozen sections for the confirmation of various tissues in the parathyroid region. Am J Clin Pathol 118:895–902
Roth SI, Marshall RB (1969) Pathology and ultrastructure of the human parathyroid glands in chronic renal failure. Arch Intern Med 124:397–407
Krause MW, Hedinger CE (1985) Pathologic study of parathyroid glands in tertiary hyperparathyroidism. Hum Pathol 16:772–784
Allen TB, Thorburn KM (1981) The oxyphil cell in abnormal parathyroid glands. A study of 114 cases. Arch Pathol Lab Med 105:421–427
Seethala RR, Richmond JA, Hoschar AP, Barnes EL (2009) New variants of epithelial-myoepithelial carcinoma: oncocytic-sebaceous and apocrine. Arch Pathol Lab Med 133:950–959
Snover DC, Foucar K (1981) Mitotic activity in benign parathyroid disease. Am J Clin Pathol 75:345–347
Schantz A, Castleman B (1973) Parathyroid carcinoma. A study of 70 cases. Cancer 31:600–605
Tominaga Y, Tsuzuki T, Matsuoka S et al (2008) Expression of parafibromin in distant metastatic parathyroid tumors in patients with advanced secondary hyperparathyroidism due to chronic kidney disease. World J Surg 32:815–821
Stojadinovic A, Hoos A, Nissan A et al (2003) Parathyroid neoplasms: clinical, histopathological, and tissue microarray-based molecular analysis. Hum Pathol 34:54–64
Yip L, Seethala RR, Nikiforova MN et al (2008) Loss of heterozygosity of selected tumor suppressor genes in parathyroid carcinoma. Surgery 144:949–955
Kilgo MS, Pirsch JD, Warner TF, Starling JR (1998) Tertiary hyperparathyroidism after renal transplantation: surgical strategy. Surgery 124:677–683 discussion 683–684
Moore C, Lampe H, Agrawal S (2001) Predictability of hypocalcemia using early postoperative serum calcium levels. J Otolaryngol 30:266–270
Kestenbaum B, Andress DL, Schwartz SM et al (2004) Survival following parathyroidectomy among United States dialysis patients. Kidney Int 66:2010–2016
Abdelhadi M, Nordenström J (1998) Bone mineral recovery after parathyroidectomy in patients with primary and renal hyperparathyroidism. J Clin Endocrinol Metab 83:3845–3851
Chou FF, Chen JB, Lee CH, Chen SH, Sheen-Chen SM (2001) Parathyroidectomy can improve bone mineral density in patients with symptomatic secondary hyperparathyroidism. Arch Surg 136:1064–1068
Collaud S, Staub-Zahner T, Trombetti A et al (2008) Increase in bone mineral density after successful parathyroidectomy for tertiary hyperparathyroidism after renal transplantation. World J Surg 32:1795–1801
Yano S, Sugimoto T, Tsukamoto T et al (2003) Effect of parathyroidectomy on bone mineral density in hemodialysis patients with secondary hyperparathyroidism: possible usefulness of preoperative determination of parathyroid hormone level for prediction of bone regain. Horm Metab Res 35:259–264
Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B (2007) Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol 18:2401–2407
Barenbrock M, Hausberg M, Kosch M, Kisters K, Hoeks AP, Rahn KH (1998) Effect of hyperparathyroidism on arterial distensibility in renal transplant recipients. Kidney Int 54:210–215
Lewanczuk RZ, Benishin CG, Shan J, Pang PK (1994) Clinical aspects of parathyroid hypertensive factor. J Cardiovasc Pharmacol 23(Suppl 2):23–26
Tzanno-Martins C, Futata E, Jorgetti V, Duarte AJ (2000) Restoration of impaired T-cell proliferation after parathyroidectomy in hemodialysis patients. Nephron 84:224–227
Yasunaga C, Nakamoto M, Matsuo K, Nishihara G, Yoshida T, Goya T (1999) Effects of a parathyroidectomy on the immune system and nutritional condition in chronic dialysis patients with secondary hyperparathyroidism. Am J Surg 178:332–336
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was written by members and invitees of the International Head and Neck Scientific Group (http://www.IHNSG.com).
Rights and permissions
About this article
Cite this article
Madorin, C., Owen, R.P., Fraser, W.D. et al. The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol 269, 1565–1576 (2012). https://doi.org/10.1007/s00405-011-1833-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1833-2