Abstract
The addition of whole body positron emission tomography (PET) to the investigation of patients with newly diagnosed head and neck squamous cell carcinoma (HNSCC) was assessed over a 6-month period. Staging investigations included laryngoscopy, oesophagoscopy, CXR, CT and MRI. In addition, all patients had an extended-field (whole body) FDG-PET scan and were restaged. Standardised Uptake Values (SUV) were used to measure FDG uptake. SUV levels above 5 were considered indicative of the presence of tumour, values below 3 indicative of benign aetiology and values equal to and between 3 and 5 were considered equivocal. Forty-eight consecutive patients with biopsy proven HNSCC were included for study. Three patients presenting with neck disease had unknown primary tumours. Of the remaining 45 patients, CT scan correctly identified 40 of the primary tumours (89%). MRI and PET both identified 41 primary tumours (91%). Thirty-two patients underwent neck dissection. Of these patients 12 had pathologically N0 necks and 20 had positive nodal disease. CT scan and MRI each correctly staged pN0 necks in 10 of 12 patients (83%) whereas PET alone had a lower true negative rate of 8 out of 12 patients (67%). PET correctly staged the N+ necks in 14/20 patients (70%) versus 12/20 (60%) for MRI, and 8/20 (40%) for CT alone. All four patients who were judged to have distant metastases by PET had these metastases deemed negative by other investigation. None of the three imaging modalities was able to identify the tumour site in the three patients with unknown primaries. In conclusion, although PET has got a higher sensitivity in detecting nodal disease, it has only slightly improved the classification of N+ necks. The findings of this study cast doubt on the merit of routine addition of PET to the current investigative protocols for HNSCC patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accurate staging of patients with head and neck cancers is essential for selecting appropriate therapy and giving accurate estimates of prognosis. Clinical examination, computed tomography (CT), and magnetic resonance imaging (MRI) have been the standard methods for assessment of this patient group but these techniques are limited in their diagnostic accuracy. There is growing evidence that PET imaging is a valuable additional tool in the evaluation of patients with head and neck carcinomas [7, 13, 17]. PET uses the radionuclide glucose analog fluorine –18 fluorodeoxyglucose (FDG) to detect tumours by assessment of the increased glucose metabolism of cancer cells compared with normal tissue. PET scanning reportedly improves detection of occult cervical lymphatic disease, distant metastasis and assist in the localisation of unknown primary carcinomas of the head and neck region [7, 13, 17]. To measure the uptake of FDG by malignant tissue, Standardised Uptake Value (SUV) has been widely used to differentiate the malignant from non malignant tissue [17, 18]. High values are expressed in many malignant tumour. Although useful, SUV in malignant and non malignant tissues overlap and provide only semiquantative analysis [27]. Moreover, there is no evidence from the published literature of an objective level or cut-off SUV to differentiate the malignant from non-malignant tissues.
The objective of this prospective study was to assess the impact of the addition of whole body PET scanning to our standard investigation protocol for new patients presenting with head and neck squamous cell carcinoma (HNSCC). Specifically the differences in classification for each of the tumour, node and metastasis categories were compared. In addition, the impact of PET scanning on patient management was assessed and recommendations for SUV diagnostic threshold to improve accuracy are described.
Materials and methods
Consecutive newly diagnosed patients with HNSCC at St James’s head and neck cancer unit from July 2003 to December 2003 were included in this prospective study. Patients with previous diagnosis or treatment of HNSCC were excluded. After endoscopic evaluation and histological-confirmed diagnosis, a standard pre-therapeutic investigation was used on all patients. This included chest radiographs and serum liver enzyme assay. The UICC manual of staging of cancer was used for classification and staging [24]. All patients had CT scan of the head and neck, thorax and upper abdomen and MRI of the head and neck region. CT scans were performed with a multislice scanner (Somatom Sensation 16; Siemens, Germany) with a 90 ml intravenous contrast injected at the rate of 3.5 ml/s. Axial and coronal slices were made with a slice thickness of 5 mm. MRI scans were obtained with a 1.5 T magnet (Magnetom Symphony; Siemens, Germany). T1 and T2-weighted images in the axial and coronal planes were obtained in 5 mm slices. Ten ml of gadolinium was injected intravenously as a contrast. CT and MRI criteria for positive nodal metastasis include nodes equal to or more than 10 mm in size with central lucency and indistinct irregular margins [17, 18].All patients had extended-field (whole body) FDG-PET study performed prior to therapy. PET imaging was performed with a full multi ring PET scanner (General Electric® ADVANCE GEMS, Milwaukee, USA). FDG was administered to fasting patients at a weight-dependent dose of between 370 and 410 MBq. Patients were instructed to avoid exercise and minimise voice use for 60 min post FDG injection. Scanning began 1 h after FDG administration and included the area from mid-thigh to crown of skull. All images were processed using iterative reconstruction and transmission attenuation correction and displayed in three orthogonal planes (transaxial, coronal, and sagittal) and in cinematic three-dimensional (MIP) display. One nuclear medicine physician (GD) evaluated all the FDG-PET images.
In those patients having primary surgery, following enbloc resection of the surgical specimen, the tissue was pinned in a fresh state on a corkboard, labelled and sent fresh for histopathological analysis. In the pathology department, all nodes were dissected, sliced at 3 mm intervals, sampled, stained and documented. The primary specimen, together with the associated neck dissection specimen was dissected in a fresh state to facilitate nodal identification. The number of nodes and presence or absence of metastasis was recorded for each site of the neck dissection, to allow correlation with imaging investigation. The results of CT, MRI and PET were compared with the final histopathological findings.
Region of interests (tumours or lymph nodes) were identified on the images and subjectively characterized in relation to normal anatomy and the uptake of FDG in surrounding tissues. FDG uptake was expressed as the standardized uptake value (SUV), using the formula:
The PET criteria for positivity were high SUV levels or intense radioactivity in areas normally exhibiting low or no activity. From literature review, empirical SUV levels indicative of benign or malignant disease have been established and used within this establishment. Areas of maximum SUV values below 3 are considered to be of benign aetiology whilst levels above 5 are more likely to be indicative of malignancy. Levels equal to, and between 3 and 5 are considered equivocal. This study looks at validity of these maximum SUV bands as indicators of malignant disease.
The diagnostic accuracy of PET was compared with that of CT and MRI. Tumour, node and metastasis classification were compared individually and the overall effect of PET on staging and treatment selection was studied. Patients were followed for evidence of recurrent disease for a minimum of 9 months.
Statistical analysis including sensitivity, specificity, positive and negative predictive values was performed using the SAS/TRAS 6 software (SAS Institute, Cary, NC, USA).
Results
Forty-eight consecutive patients were included for study. There were 42 men and 6 women. The mean age was 56 years (range 32–80 years). Forty-five patients had histological confirmation of their primary HNSCC while three patients had cervical metastatic SCC of unknown primary. Of the 48 patients, 33 were treated by combined surgery and post-operative radiotherapy, three by surgery alone and 12 patients by primary chemo-radiation. Tumour details before the addition of PET are shown in Table 1.
CT scan correctly identified 40 of 45 primary tumours (89%). MRI and PET each identified 41 out of 45 primary tumours (91%).
Thirty-two patients underwent neck dissection. 17 had bilateral neck dissections. Twelve of these 32 patients had pathologically negative neck nodes (pN0) and 20/32 (63%) patients had pathologically positive neck nodes (pN+). Of the 20 patients with histologically proven cervical nodal disease, CT correctly classified 8 of 20 (40%) patients. MRI correctly classified 11 patients (55%). PET, however, correctly classified cervical nodal disease in 14 patients (70%). Table 2 shows CT, MRI and PET classification results of cervical nodal metastasis together with sensitivity, positive predictive values and accuracy of nodal classification for the three diagnostic modalities.
CT and MRI were equal in correctly staging pN0 necks in 10 of 12 patients (83%), while PET correctly staged 9 of 12 patients (75%) with node negative disease.
Nodal positivity status before and after the addition of PET scan was also studied. The classification of nodal metastasis was changed in eight patients (Table 3). The correlation with histological examination and the impact on management are also shown in Table 3.
Three patients with cervical metastatic disease had unknown primary tumours. All three imaging modalities failed to detect a primary tumour in any of these cases.
In four of the 48 patients (8%) areas of increased FDG uptake consistent with distant thoracic metastasis were identified. All four patients eventually had these areas deemed negative for distant metastasis following further investigations including bronchoscopic and fluoroscopically guided biopsy together with serial thoracic CT. Clinical follow up for more than 18 months of these patients confirm that they are without evidence of thoracic metastatic disease. The findings in these cases and comparison with CT scans are summarized in Table 4.
FDG uptake by avid tissues and lymph nodes was attributed a semiquantative value calculated by the SUV formula in the methods section. The maximum SUV in the primary tumours identified by PET (41 tumours) ranged from 5.6 to 30, with a mean value 14.4 (Fig. 1).
Table 5 shows the sensitivity and specificity of four selected maximum SUV thresholds for nodal metastasis.
The confirmed discriminatory power of the selected maximum SUV thresholds (<3–5; >5) was evaluated in the nodal metastasis group using a receiver operating characteristics (ROC) plot demonstrated in Fig. 2 [4].
Discussion
Positron emission tomography (PET), a relatively new imaging modality, has been shown to improve the clinical staging of head and neck cancers [7, 13, 17]. The use of whole-body PET imaging readily permits evaluation of the entire body for metastatic disease in contrast to conventional CT and MRI locoregional scan protocols. In the present study, PET was prospectively incorporated into patients’ clinical staging pathway for patients with HNSCC.
PET was able to detect and localise primary tumours in 41 out of 45 evaluated patients. The four primaries that PET failed to identify were early stage tumours: two were T1glottic, one was T1 supraglottis and one was T2 pyriform fossa. CT and MRI similarly failed to detect the first three of these tumours but did detect the T2 pyriform fossa SCC. The sensitivity of PET and MRI each was 91% (41/45) and for CT scanning was 89% (40/45). Thus PET was no better than CT or MRI in detecting primary tumours in the current study.
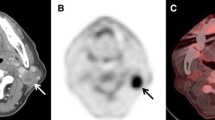
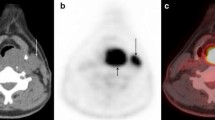
Accurate establishment of locoregional lymph node disease status is a major determinant of treatment selection and prognosis in HNSCC [12]. The incorrect neck nodal staging by PET in 6 of 20 pN+ patients (Table 2) comprised five understaging events and a single neck status overstage. Of the five patients erroneously understaged by PET, two were staged as node negative, and in the other three, PET failed to detect contralateral lymph node metastases. This later, false negative result of PET may be attributable to the small volume and intrinsic low glycolytic activity of metastatic tissue within cervical nodes, both essential for visualising FDG uptake [8]. CT scan correctly staged only eight patients and MRI 11 of the 20 pN+ patients. All remaining patients were erroneously understaged. This limitation of CT and MRI scanning in detecting metastatic lymph nodes could be attributed to the restricted criteria of positivity already described above in the method section. This was demonstrated clearly in 3 cases (see Table 3). In case 1 both CT and MRI scanning failed to detect any enlarged lymph nodes in the neck, however, PET showed two ipsilateral hot nodes at level II with SUV above 5. Histology showed two positive lymph nodes at level II out of 59 nodes in neck dissection specimen. In case 4 (Table 3) CT scan showed four separate lymph nodes ranging in size from 3 to 6 mm. The radiologist could not call them metastases as they are sub centimetre and they were also not filling other criteria for positivity on CT scan. In contrast PET showed the presence of a hot node at level III, this was confirmed positive by pathological examination. In case 6 (Table 3) the CT scan showed only bilateral submental and submandibular lymph nodes (level I) less than one centimetre and they were not filling the other criteria for positivity. PET Showed two enlarged ipsilateral cervical lymph nodes at level IV in the neck with high SUV. The histology confirmed the positivity of these lymph nodes, however, the nodes in level I which were suspicious on CT were negative by pathological examination.
The current study showed that PET was more sensitive (73%) than CT (42%) or MRI scans (55%) alone in detection of involved lymph nodes (Table 2). These results are comparable with previous reports. Bailet et al. studied eight neck dissection specimens and found sensitivity for PET of 71%, while MRI and CT each had lower sensitivities of 58% [6]. Jabour et al. [11] claimed an almost identical sensitivity of 74% in respect of nodal positivity detection by PET in 12 patients with oral SCC. In a group of 22 patients with HNSCC, Laubendacher et al. reported a sensitivity of 89% for PET compared with 72% for MRI [17].
Of the 12 patients in the current study with pN0 necks, PET correctly staged 9, whilst both CT and MRI correctly staged 10 patients each (Table 2). Three of the 12 pN0 patients were incorrectly deemed N + by PET. Therefore, PET was less accurate than either CT or MRI for classification of N0 necks. PET has been shown to be susceptible to false positive results [23]. Foci of FDG uptake within non tumour such as inflammatory tissue and abscesses limit the value of FDG tracer in accurate detection of tumour tissue [8]. Stoeckli et al. [25] studied 12 patients with oral and oropharyngeal SCC and no clinical or radiological evidence of lymph node metastasis. In their studies, all patients had PET studies performed 24 h before surgery and sentinel lymph node (SLN) biopsy performed intraoperatively. The authors reported a disappointing sensitivity of 25% for PET and a specificity of 88%. PET was thus ascribed no primary role in the staging of N0 necks. However, Braams et al. [8] reported a sensitivity of 100% for PET in a study that similarly included 12 patients with SCC of oral cavity and clinically N0 necks. This higher sensitivity rate purports to claim an important role for PET in detection and staging accuracy of occult nodal disease.
To study the impact of PET on nodal classification, we reviewed nodal metastasis pre and post addition of PET (Table 3). Eight patients (25%) had different nodal classification after PET imaging seven from the pN+ group and one from the pN0 group. Six were upstaged and two down staged. Three patients (9%) out of these eight patients had their surgical treatment changed; two from the pN+ group and one from the pN0 group. In case 1 (Table 3) a T3 tongue, both CT and MRI failed to show any nodal metastasis (N0), however, PET showed ipsilateral nodal metastases (N2B).
Therefore the intended treatment plan of unilateral selective neck dissection was changed and the patient had a modified radical neck dissection on the tumour side and selective neck dissection on the other side. The PET findings correlated with the final pathological staging. In the second patient (case 6 in Table 3) a T2 tongue, there was no nodal metastasis shown on either CT or MRI (N0). However PET scanning showed ipsilateral cervical disease (N2B). Therefore the treatment plan was altered from unilateral selective neck dissection to unilateral modified radical neck dissection. This also correlated pathological classification. In the third case of the pN0 group (case 8) PET showed bilateral nodal metastasis .Subsequently the intended plan of bilateral selective neck dissection was changed to bilateral modified radical neck dissection. Histopathological examination of the specimens showed no nodal disease on either side.
It has been reported that ultrasound (US) is comparable to CT scan in identification of metastatic cervical lymph nodes [2, 20]. The method is widely available, well tolerated by patients and economical. There is no need for contrast medium and there is no exposure to radiation. However, no standardized permanent documentation is obtained with US, so that the accuracy fully depends on the examiner [28]. Furthermore, US can not depict or stage most primary tumours, often necessitating the use of CT or MR anyway. Retropharyngeal nodes can also not be detected with US.
Because of these limitations of US, a combination of US with fine needle aspiration cytology (FNAC) is considered the most accurate technique for detecting or excluding the presence of nodal metastasis preoperatively [5,28]. However, micrometastasis can be missed, material aspirated could be not sufficient for diagnosis and aspiration can be obtained from a wrong node [28]. Therefore, there might be considerable false negative results with this method. Consequently it remains a difficult decision not to treat a patient in whom there is a suspicious palpable lymph node with a benign cytological finding.
In our clinical practice US is not routinely used for the investigation of cervical nodal metastasis. It is labour intensive and the patient is sure to have CT scan anyway which is readily available and can view both primary tumour and the nodal metastasis. In our institute US-guided FNAC is predominantly used as a problem-solving tool. In a situation where cervical lymph nodes are deep and not accessible and the patient has unknown primary or there is a suspicion of recurrent deep metastatic lymph node then US-guided FNAC can be very helpful.
Positron emission tomography appeared to detect distant metastases in four of the current study patients (Table 4). These were eventually diagnosed as non malignant (false positives). CT scanning was truly negative in three patients and therefore CT, in this study, was more useful than PET in evaluating apparent distant pulmonary metastases. Keyes et al. [14], reported on nine patients with areas of increased intrathoracic FDG uptake. Six of these were false-positive (67%). Of the three confirmed true positive lesions, two had been detected by conventional imaging. In one case the PET study detected a significant lesion not found by routine evaluation, resulting in a reported 2% case finding yield. The report concluded that there appeared to be no compelling evidence to include the entire chest region in the PET scan volume during the evaluation of head and neck cancers. Wax et al. [29], reporting on 54 patients with previously untreated SCC of the upper aero digestive tract described positive lung findings on PET in 10 patients. The authors determined lower accuracy and positive predictive values for PET than CT scan, but higher sensitivity.
The methodology of SUV determination suffers from known quantitative inaccuracies [27]. Subjection to statistical scrutiny of SUV as a diagnostic tool and prognostic indicator in HNSCC has only recently been reported [3, 21].
All the maximum SUV values of primary tumours detected by PET in this study were above 5.6 (Fig 1). The absence of false positive PET findings in primary tumours rendered statistical analysis on the sensitivity and specificity of SUV thresholds unsatisfactory.
Thirty two patients underwent neck dissections; 17 were bilateral, yielding 49 neck sides for analysis. If the value of 2.9 (the lowest recorded maximum SUV for true nodal metastases) is chosen as the threshold level maximum SUV indicating test positivity, PET would have a sensitivity of 100% but only 20% specificity. The cross-directional influence of SUV alterations on sensitivity and specificity is evident from results in Table 5. Raising the maximum SUV threshold for malignancy detection to 3.2 reduces the sensitivity to 97% and increases the specificity to 40%. For this study, this improvement in specificity and slight reduction in sensitivity appears to make ≥3.2 maximum SUV thresholds a reasonable indicator of PET test nodal positivity for tumour. From these results (Table 5), it would appear that our previously selected SUV bands of(1) <3=unlikely tumour, (2) 3–5=equivocal for tumour and (3) >5=tumour positive, appear validated for the detection of malignant nodal disease. SUV results in the cervical region between 3 and 5 are equivocal and must be further investigated with the ongoing suspicion of underlying malignancy.
The addition of PET to established conventional clinical and radiological investigative protocols for cervical metastatic SCC of unknown primary remains the subject of considerable debate [13, 19]. Three patients in this study who had SCC primary tumours metastatic to the neck went undetected by CT, MRI and PET. Mendenhall et al. [19] evaluated radiological modalities in the detection of primary tumour sites in 130 patients with SCC metastatic to cervical lymph nodes. PET identified a small subset of primary lesions that were not apparent on standard clinical examination and/or radiological evaluation with CT and/or MRI. They reported the PET to be of very modest value which did not warrant inclusion in routine diagnostic workup of patients with cervical metastatic SCC of unknown primary. The study by Fogarty et al. consisting of 21 patients claimed PET added little to occult primary detection [10]. PET detected a potential primary site in eight patients. However, only one of those eight primary sites was pathologically confirmed. Evidence supporting increased PET usage is provided by Stokkel et al. [26], with a 50% success rate in unknown primary detection. Similarly, Aasar et al. [1], in their series of 15 patients with unknown primaries reported a 70% positive biopsy rate from targeted FDG weighted tissues. Theories as to why unknown primaries and not their nodal metastases may elude PET detection include insufficient primary tumour versus background FGD uptake; intrinsic differences in primary- versus nodal-tumour metabolism; previous primary treatment or surgical removal and spontaneous primary involution or regression [15].
Combined PET/CT scanners have become commercially available in the past few years. It fuses the anatomic data of CT with functional data of PET. The fusion of FDG-PET with CT scan has been shown to be more accurate than PET or CT alone for the detection of malignancy in head and neck [9, 16, 22]. Unfortunately there was no PET/CT machine available in our department at the time we did this study. However, CT scan films were available for the radiologist when he reported on PET scanning.
Conclusion
Although the number of patients in this study is relatively small, this study demonstrates PET to be comparable to current conventional imaging modalities in detecting primary tumours. The high rate of false positive (upstaging) results of PET in nodal metastasis highlights the higher sensitivity of PET in detecting nodal disease. However, PET has only slightly improved the classification of N+necks. At present, PET has no considerable role to play in N0 neck imaging protocols. This study shows PET to be less sensitive than both CT and MRI in detecting occult nodal disease. PET proved to be disappointingly similar to CT and MRI in an attempted identification of a small number of unknown primaries. PET was not reliable in detecting distant metastasis, as the rate of false positive findings was high. However, interpretation of results is limited by the small number of study patients with distant metastases.
These findings cast doubt on the merit of the routine addition of PET to the current investigative radiology protocols for presenting HNSCC patients.
Maximum SUV currently appears to be a reasonable index of malignancy in HNSCC primary and metastatic tumour. This study established a lower maximum SUV threshold of 3.2 for nodal tumour in a series of 41 neck side dissections. The authors recommend that maximum SUV equal to and between 3 and 5 are equivocal and should be interpreted with caution. Values below 3 are unlikely to represent tumour and those above 5 are most likely indicative of tumour activity.
References
Aassar OS, Fischbein NJ, Caputo GR, Kaplan MJ, Price DC, Singer MI, Dillon WP, Hawkins RA (1999) Metastatic head and neck cancer: role and usefulness ofFDG PET in locating occult primary tumours. Radiology 210:177–181
Akogula E, Dutipek M, Bekis R, Degirmenci B, Ada E, Guneri A (2005) Assessment of cervical lymph node metastasis with different imaging methods in patients with head and neck squamous cell carcinoma. J Otolaryngol 34:384–394
Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P (2003) Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18f]fluoro-2-deoxy-d-glucose. Int J Radiat Onco Biol Phys 59:1295–1300
Altman DG, Bland JM (1994) Diagnostic tests 3: receiver operating characteristic plots. BMJ 309:188–189
Atula TS, Grenman R, Varpula MJ, Kurki TJI, Klemi PJ (1996) Palpation, ultrasound, and ultrasound- guided fine-needle aspiration cytology in the assessment of cervical lymph node status in head and neck cancer patients. Head Neck 18:545–551
Bailet JW, Abemayer E, Jabour BA, Hawkins RA, Ho C, Ward PH (1992) Positron emission tomography: A new precise imaging modality for detection of primary head and neck tumours and assessment of cervical adenopathy. Laryngoscope 102:281–288
Bailet JW, Sercarz JA, Abemayer E, Anzai Y, Lufkin RB, Hoh CK (1995) The use of positron emission tomography for early detection of recurrent head and neck squamous cell carcinoma in postradiotherapy patients. Laryngoscope 105:135–139
Braams W, Pruim J, Freling NM, Nikkels PJ, Roodenburg JN, Boering G, Vaalburg N, Vermey A (1995) Detection of lymph node metastases of squamous cell cancer of head and neck with FDG-PET and MRI. J Nucl Med 36:211–216
Branstetter BF, Blodget TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, Meltzer CC (2005) Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology 235:580–586
Fogarty BG, Peters LJ, Stewart J, Scott C, Rischin D, Hicks RJ (2003) The usefulness of fluorine 18-labelled deoxyglucose positron emission tomography in the investigation of patients with cervical lymphadenopathy from an unknown primary tumour. Head Neck 25:138–145
Jabour BA, Choi Y, Hoh CK, Rege SD, Soong JC, Lufkin RB, Hanafee WN, Maddahi J, Chaiken L, Bailet J (1993) Extracranial head and neck PET imaging with 2-(F-18)Fluoro-2-Deoxy-D-Glucose and MR imaging correlation. Radiology 186:27–35
Jones AS, Roland NJ, Field JK, Philips DE (1994) The level of cervical lymph node metastases; their prognostic relevance and relationship with head and neck squamous cell carcinoma primary sites. Clin Otolaryngol 19:63–69
Jungehülsing M, Scheidhauer K, Damm M, Pietrzyk U, Eckel H, Schicha H, Stennert E (2000) 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography is a sensitive tool for the detection of occult primary cancer (carcinoma of unknown primary syndrome) with head and neck lymph node manifestation. Otolaryngol Head Neck Surg 123:294–301
Keyes JW Jr, Watson NE Jr, Williams DW 3rd, Greven KM, McGuirt WF (1997) FDG PET in head and neck cancer. Am J Roentgenol 169:1663–1669
Keyes JW Jr, Chen MYM, Watson NE Jr, Greven KM, McGuirt WF, Williams DW (2000) FDG PET evaluation of head and neck cancer: Value of imaging the thorax. Head Neck 22:105–110
Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW (2005) F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck 27:494–502
Laubenbacher C, Saumweber D, Wagner-Manslau C, Kau RJ, Herz M, Avirl N, Ziegler S, Kruschke C, Arnold W, Schwaiger M (1995) Comparison of fluorine-18-fluorodeoxyglucose PET, MRI and endoscopy for staging head and neck squamous-cell carcinomas. J Nucl Med 36:1747–1757
McGuirt WF, Williams DW, Keyes JW, Greven KM, Watson NE, Geisinger KR, Cappellari JO (1995) A comparative diagnostic study of head and neck nodal metastases using positron emission tomography. Laryngoscope 105:373–375
Mendenhall WM, Mancuso AA, Parsons JT, Stringer SP, Cassissi NJ (1998) Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Head Neck 20:739–744
Sarvanan K, Bapuraj JR, Sharma SC, Radotra BD, Khandelwal N, Suri S (2002) Computed tomography and ultrasonographic evaluation of metastatic cervical lymph nodes with surgicoclinicopathologic correlation. JLO 116:194–199
Schmidt M, Schmalenbach M, Jungehulsing M, Theissen P, Dietlein M, Schroder U, Eschner W, Stennert E, Schicha H (2004) 18F-FDG PET for detecting recurrent head and neck cancer, local lymph node involvement and distant metastases. Comparison of qualitative visual and semiquantitative analysis. Nuklearmedizin 43:91–101 and quiz 102–104
Schoder H, Yeung HW, Gonen M, Kraus D, Larson SM (2004) Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology 231:65–72
Schwartz DL, Rajendran J, Yueh B, Coltera M, Anzai Y, Krohn K, Eary J (2003) Staging of head and neck squamous cell cancer with extended-field FDG-PET. Arch Otolaryngol Head Neck Surg 129:1173–1178
Sobin LH, Wittekind CH (2002) International Union against Cancer (UICC): TNM Classification of malignant tumours., 6th edn. Wiley-Liss, New York
Stoeckli SJ, Steinert H, Pfaltz M, Schmid S (2002) Is there a role for positron emission tomography with 18F-fluorodeoxyglucose in the initial staging of nodal negative oral and oropharyngeal squamous cell carcinoma. Head Neck 24:345–349
Stokkel MP, Terhaard CH, Hordijk GJ, Van Rijk PP (1999) The detection of unknown primary tumours in patients with cervical metastases by dual head positron emission tomography. Oral Oncol 35:390–394
Sundaram SK, Freedman NM, Carrasquillo JA, Carson JM, Whatley M, Libutti SK, Sellers D, Bacharach SL (2004) Simplified kinetic analysis of tumour 18F-FDG uptake: adynamic approach. J Nucl Med 45:328–333
van den Brekel MWM, Castelijns JA, Stel HV, Golding RP, Meyer CJL, Snow GB (1993) Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastasis : a prospective comparative study. Eur Arch Otorhinolaryngol 250:11–17
Wax MK, Myers LL, Gabalski EC, Husain S, Gona JM, Nabi H (2002) Positron emission tomography in the evaluation of synchronous lung lesions in patients with untreated head and neck cancer. Arch Otolaryngol Head Neck Surg 128:703–707
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hafidh, M.A., Lacy, P.D., Hughes, J.P. et al. Evaluation of the impact of addition of PET to CT and MR scanning in the staging of patients with head and neck carcinomas. Eur Arch Otorhinolaryngol 263, 853–859 (2006). https://doi.org/10.1007/s00405-006-0067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-006-0067-1