Abstract
Purpose
Our aim was to investigate the diagnostic accuracy of fluorine-18-labeled fluorodeoxyglucose positron emission tomography computed tomography (FDG-PET/CT) relative to CT for detecting neck lymph node metastases in patients with squamous cell carcinoma (SCC) of the hypopharynx, oropharynx, and larynx.
Methods
Thirty-four patients with SCC of the hypopharynx (n = 20), oropharynx (n = 5), and larynx (n = 9) who underwent neck dissection (29 bilateral, 5 unilateral; a total of 355 nodal levels) were assessed. Two observers determined the long-axis diameter and maximum standardized uptake value (SUVmax) of all visible neck nodes. Results of FDG-PET/CT were compared with those of corresponding histopathologic examinations according to the neck-level system.
Results
Histopathology revealed metastases in 70 of 355 nodal levels. Using a best discriminative SUVmax cutoff of 3.65, sensitivity, specificity, and accuracy of FDG-PET/CT on a level-by-level basis were 72.9, 96.8, and 92.1 %; those for CT were 52.9, 98.6, and 89.6 %. Differences in sensitivity and accuracy were significant (p < 0.01). The best cutoff SUVmax on the ipsilateral side was 4.61, with corresponding figures of 81.6, 100, and 94.7 %; that on the contralateral side was 2.41, with figures of 60, 88.4, and 85.4 %.
Conclusion
FDG-PET/CT with SUVmax is useful for preoperative evaluation of neck-node metastasis from SCC of the pharynx and larynx, especially on the ipsilateral side.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pretreatment assessment of neck lymph node metastasis is important for therapeutic planning and prognostication in patients with head and neck squamous cell carcinoma (HNSCC) [1]. Preoperative nodal status is usually evaluated by clinical examinations such as palpation, ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI). However, CT and MRI, which evaluate morphologic parameters such as node size, internal architecture, and contrast enhancement pattern, have limited value for this purpose [2]. At present, neck dissection with histologic examination of lymph nodes is still the most reliable staging procedure. However, it is unavoidably invasive, and a noninvasive procedure capable of providing high-quality prognostic data approaching this gold standard would be of immense value.
Positron emission tomography (PET) using the glucose analog fluorine-18-labeled fluorodeoxyglucose (FDG) is a functional imaging modality that provides information about tissue glucose metabolism. Integrated positron emission tomography (PET)/CT has been applied successfully for staging, restaging, therapy evaluation, and prognostication of HNSCC, and recent reports suggest that it is also useful for evaluating nodal involvement. There is growing evidence that FDG-PET or PET/CT is a more reliable and accurate imaging tool for evaluating neck lymph node metastases than CT, although their diagnostic value remains controversial [3–15]. Moreover, most previous assessments of these modalities have been done in patients with cancers of the oral cavity (tongue, gum, floor of the mouth, and buccal mucosa) [3, 5, 13] or cancers affecting the oral cavity, pharynx, and larynx [4, 6–12, 14, 15]; few reports focus on patients with cancers of the pharynx and larynx only. Therefore, the purpose of our study was to retrospectively investigate the diagnostic accuracy of FDG-PET/CT relative to CT for detecting neck lymph node metastases in patients with squamous cell carcinoma (SCC) of the hypopharynx, oropharynx, and larynx by employing histologic evaluation of neck dissection as the reference standard.
Materials and methods
Patients
This was a retrospective study approved by our institutional review board. The study involved 34 patients (29 men, five women; average age at diagnosis 69.1 years, range 50–91 years) with biopsy-proven SCC of the pharynx and larynx. Primary tumor sites were the hypopharynx (n = 20), oropharynx (n = 5), and larynx (n = 9). All patients underwent resection of the primary tumor and neck-node dissection within 4 weeks after undergoing FDG-PET/CT examinations at our institution between December 2011 and October 2014; all underwent unilateral (n = 5) or bilateral (n = 29) neck dissection. Patient characteristics are shown in Table 1.
Image analysis
Two board-certified observers (observer A, a double board-certified nuclear medicine physician and radiologist with 10 years of experience in oncologic FDG-PET/CT; observer B, a board-certified head and neck imaging radiologist with 4 years of experience in oncologic FDG-PET/CT) consensually reviewed neck-node findings on PET/CT images. According to the neck-level system defined by the American Joint Committee on Cancer and the American Academy of Otolaryngology [16], the longest axial diameter and the maximum standardized uptake value (SUVmax) of visible neck lymph nodes were measured in transaxial PET/CT images. No FDG accumulation to the node was defined as N0. SUVmax was calculated for quantitative analysis of tumor FDG uptake, as follows:
where C is the tissue activity concentration measured by PET, and ID is the injected dose.
On CT images, neck nodes were considered to contain metastasis if their longest axial diameter was >15 mm for levels I and II, >10 mm for levels III–V, or >8 mm in the parapharyngeal and retropharyngeal space [16, 17].
If there were multiple lymph nodes at a given level, the largest one evident on CT or the one with the highest SUVmax on PET/CT was assessed.
Surgical procedure and histology
Neck dissection was planned by our head and neck surgical team based on clinical and imaging findings [18]. Supraomohyoid neck dissection (SOHND, levels I–III) was performed for patients who were node negative in the neck or who had a single positive node in the upper neck. Extended supraomohyoid neck dissection (extended SOHND, levels I–IV) or modified radical neck dissection (MRND, levels I–V) was performed for patients with more than one involved node or with extracapsular nodal spread, depending on the extent of the cervical adenopathy. Bilateral neck dissection was performed for patients in whom the primary tumor crossed the midline or those considered likely to have node metastases in the contralateral neck. The operating surgeon labeled the primary tumor and neck dissection specimens to allow reference to the schema used to interpret the FDG-PET/CT studies. Lymph nodes and tumors were dissected from the specimens and stained with hematoxylin and eosin (H&E) for histologic analysis. Serial histologic sections were used. An experienced pathologist examined the specimens and recorded the number, size, and capsular penetration of affected nodes.
Statistical analysis
On the basis of the neck-level system, we compared results of preoperative examinations using FDG-PET/CT and the CT component with those of the corresponding histopathologic examinations. Because precise spatial correlation between PET/CT and histopathology is impossible, analysis was restricted to nodal levels II–VI on the right and left sides. If PET/CT findings were suggestive of metastasis, and if histopathology showed at least one lymph node with metastasis at a given nodal level in the neck, a true positive finding was recorded, regardless of the number of metastatic foci at that level. SUVmax and longest axial diameter of ipsilateral and contralateral lymph nodes associated with the primary tumor were also evaluated.
Receiver operating characteristic (ROC) curve analysis was performed to evaluate whether SUVmax allowed diagnosis of nodal metastasis and to identify the best cutoff value. Analysis was performed according to patient, neck side, and lymph node levels. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated using standard statistical formulae, and the 95 % confidence interval (CI) was determined for each parameter. McNemar test was used to compare sensitivity and specificity for evaluating two diagnostic tests. Differences at p < 0.05 were considered statistically significant. All analyses were performed using the SAS software package version 9.2 (SAS Institute, Cary, NC, USA).
Results
Thirty-four patients underwent dissection of 63 neck sides (five unilateral, 29 bilateral), and 355 cervical lymph node levels were analyzed. Histopathologic analysis revealed lymph node metastases in 28 of the 34 patients (82.4 %), 38 of the 63 neck sides (60.3 %), and 70 of the 355 nodal levels (19.6 %). Neck lymph nodes were dissected at 355 neck levels (II:III:IV:V:VI:others = 122:60:60:37:42:34), and malignant cells were found at 70 neck levels (II:III:IV:V:VI:others = 29:22:11:4:2:2).
Primary tumors
All 34 primary tumors were clearly identified by FDG-PET; FDG uptake intensity in these lesions was high, with an SUVmax range of 9.41–26.48 (mean 17.61 ± 4.62). A representative case is shown in Fig. 1.
A 71-year-old man with ipsilateral neck lymph node metastasis arising from cancer of the hypopharynx. a Computed tomography (CT) of positron emission tomography (PET)/CT shows a hypopharyngeal mass (short arrow) and one swollen lymph node 17 × 22 mm in size at left level III node (long arrow), suggesting the presence of nodal cancer spread. b Fluorine-18-labeled fluorodeoxyglucose (FDG)-PET and c fused PET/CT show intense FDG uptake corresponding to the hypopharyngeal mass [short arrow; maximum standardized uptake value (SUVmax):13.91] and left level III node (long arrow, SUVmax:11.25), suggesting the presence of nodal cancer spread from hypopharynx cancer. Examination of the histopathological specimen confirmed extensive lymph node involvement by cancer in this node. Both CT and FDG-PET/CT were true-positive
Metastatic neck disease
Level-by-level analysis
The SUVmax of lymph node metastases (mean SUVmax, 7.67 ± 5.91; range 0–25.71) was significantly higher than that of benign lymph nodes (mean SUVmax, 0.53 ± 1.51: range 0–17.41) (p < 0.005). Using the best SUVmax cutoff of 3.65 for distinguishing between malignant and benign lymph nodes based on ROC curve analysis, sensitivity, specificity, and accuracy of FDG-PET/CT for identifying nodal metastases on a level-by-level basis were 72.9 (51/70), 96.8 (276/285), and 92.1 % (327/355), respectively; corresponding values for CT were 52.9 (37/70), 98.6 (281/285), and 89.6 % (318/355), respectively. Sensitivity (p = 0.00051) and accuracy (p = 0.0077) of FDG-PET/CT were significantly better than those of CT (Table 2). Two representative cases are shown in Figs. 1 and 2: Fig. 1 shows that both CT and FDG-PET gave true-positive results, whereas Fig. 2 shows that CT gave a false-negative result and FDG-PET a true-positive result.
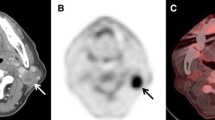
A 75-year-old man with right-sided level IIa node metastasis arising from cancer of the hypopharynx. a Computed tomography (CT) of positron emission tomography (PET)/CT shows one swollen lymph node 8 × 9 mm in longest diameter (arrow), suggesting absence of nodal cancer spread. b Fluorine-18-labeled fluorodeoxyglucose (FDG)-PET and c fused PET/CT show moderate FDG uptake [maximum standardized uptake value (SUVmax):6.49] corresponding to the right level IIa node seen in a (arrow), suggesting the presence of nodal cancer spread. Histopathological examination of the specimen confirmed extensive lymph node involvement by cancer in the right node. CT gave a false-negative result and FDG-PET/CT gave a true-positive result
Level-based analysis
For level II and III nodes, sensitivity and accuracy of PET/CT were higher than those of CT. The difference reached a significant level for level II nodes (p = 0.0044 and p = 0.023, respectively). At other levels (IV, V, VI, and others), diagnostic performances were almost the same (Table 3).
Comparison between sides ipsilateral and contralateral to primary tumor
Among the 355 neck levels at which nodes were detectable, 133 were clearly detected on the side ipsilateral to the primary tumor; 96 were detectable on the contralateral side. SUVmax of lymph nodes containing metastasis on the ipsilateral side (mean SUVmax, 8.65 ± 5.71; range 0–25.70) was significantly higher than on the contralateral side (mean SUVmax, 4.23 ± 5.13: range 0–11.25; p = 0.032). The best cutoff SUVmax for discriminating between malignant and benign nodes on the ipsilateral side was 4.61, with a sensitivity, specificity, and accuracy of 81.6 (31/38), 100 (95/95), and 94.7 % (126/133), respectively; that on the contralateral side was 2.41, with corresponding values of 60 (6/10), 88.4 (76/86) and 85.4 % (82/96), respectively ( Table 4). The longest axial diameter of lymph nodes harboring metastasis was significantly larger on the ipsilateral side (mean 17.0 ± 10.2 mm; range 4.0–41.3 mm) than on the contralateral side (mean 13.5 ± 12.3 mm; range 4.0–44.0) (p = 0.0018) (Table 5).
Side-based analysis
Side-based analysis showed that sensitivity, specificity, and accuracy of FDG-PET/CT were 89.5 (34/38), 84.0 (21/25), and 87.3 % (55/63), respectively, whereas corresponding CT values were 68.4 (26/38), 92.0 (23/25), and 77.8 % (49/63), respectively. Sensitivity (p = 0.0133) and accuracy (p = 0.0412) of FDG-PET/CT were significantly better than those of CT.
Patient-based analysis
Sensitivity, specificity, and accuracy of FDG-PET/CT on a patient basis were 92.9 (26/28), 83.3 (5/6), and 91.2 % (31/34), respectively, and corresponding figures for CT were 75.0 (21/28), 83.3 (5/6), and 76.5 % (26/34), respectively. None of these values differed significantly between the two modalities.
Discussion
The major findings of the study reported here were that: (1) FDG-PET/CT is more useful than CT for preoperative evaluation of neck lymph node metastases in patients with SCC of the pharynx and larynx, and (2) it is not easy to judge whether metastases are present in lymph nodes on the side of the neck contralateral to the primary tumor.
CT and MRI are commonly used for evaluating primary tumor and neck-node status in patients with HNSCC. These modalities characterize neck lymph nodes on the basis of morphological criteria, such as node size, presence of central necrosis, and presence of indistinct nodal margins. Reported sensitivity and specificity of CT and MRI for detecting neck lymph node metastases in HNSCC are 36–79 and 47–99 %, respectively [2, 3, 8–10, 12–15]. Doppler US with fine-needle aspiration biopsy can overcome some of these limitations, but results are dependent on the skill level of the sonographer, and using this modality may be impractical in some cases because the number of questionable nodes may be high.
Several studies have evaluated the diagnostic performance of FDG-PET or PET/CT for detecting neck lymph node metastases of HNSCC. Data from those studies demonstrated large variations in sensitivity and specificity, being 50–96 and 82–99 %, respectively [3–15]. Sun et al. [19] reviewed 24 studies of 1270 patients with HNSCC to assess nodal metastasis and reported that the mean (95 % CI) pooled per-patient, per-neck-side, and per-neck-level sensitivities/specificities of FDG-PET/CT were 91 % (82–95 %)/87 % (80–92 %), 84 % (75–90 %)/83 % (77–88 %), and 80 % (71–87 %)/96 % (94–97 %), respectively. Moreover, across 13 studies (3460 neck levels) for which per-neck-level data were available, sensitivity and specificity were 84 % (72–91 %) and 96 % (95–97 %), respectively, for FDG-PET/CT and 63 % (53–72 %) and 96 % (95–97 %) for conventional imaging (CT and MRI), respectively.
Only three studies compared the diagnostic accuracy of ipsilateral and contralateral neck lymph node staging by FDG-PET/CT in patients with HNSCC [20–22]. All concluded that the SUVmax of contralateral neck nodes was lower than that of ipsilateral neck nodes with metastasis due to their smaller size and that PET/CT had lower sensitivity for detecting contralateral than ipsilateral neck metastasis, similar to our results.
Although FDG-PET is more useful than CT for diagnosing neck lymph node metastasis, the diagnostic capability of FDG-PET is limited not only by cellular activity but also by tumor volume. FDG uptake by small deposits of tumor cells is often poorly depicted owing to partial-volume effects [23]. Moreover, its registration is limited to a certain lymph node size because the spatial resolution of recent PET scanners is technically limited to 4–5 mm. A previous study suggested that occult metastases have tumor involvement extending over only 1–2 mm [24]. Therefore, FDG-PET scanning may be unable to detect such occult nodal metastases. In the future, development of dual-time-point PET, new tumor-specific tracers, and PET scanners with a higher resolution may increase the potential to detect occult lymph node metastases.
False-positive findings were encountered in several cases in our study. The intensity of tracer uptake by inflammatory lymph nodes is virtually the same as that by metastatic lesions. Common exposure to carcinogens in tobacco smoke and alcohol may be responsible for chronic, low-level lymphadenitis in patients with head and neck cancer. In accordance with this possibility, histologic analysis of false-positive nodes demonstrated follicular and parafollicular hyperplasia of lymphoid tissue in all cases.
There were several limitations to our study. First, it had a retrospective design and involved a relatively small number of patients at a single institution. Second, as every patient was a candidate for surgery, including neck dissection, a patient selection bias was unavoidable. Third, although node-based analysis is an ideal approach, it was very difficult to correlate any given lymph node depicted in an imaging study with the same node in a neck dissection specimen. Therefore, correlation of imaging results with pathological findings on a neck-level basis may be more reasonable if it is done in terms of sensitivity and specificity. Fourth, there was the problem of underestimating SUVmax of tiny lymph nodes due to the partial-volume effect; Bundschuh et al. [25] developed a semiautomatic algorithm using CT and PET to obtain a partial-volume-corrected (PVC) SUV and a combined morphologic and functional parameter (multimodal SUV) for lymph node assessment. They also demonstrated that SUV values were increased significantly (p < 0.0005) after PVC (mean 2.8) in comparison with those before PVC (mean 1.29). If SUV 2.5 had been used as a threshold value to distinguish between benign and malignant lesions, the status of 11 of the 47 nodal lesions would have changed from benign to malignant after PVC. Fifth, given the low-dose, non-enhanced CT method we employed, visualization of nodes may have been reduced in comparison with that for diagnostic contrast-enhanced CT. Sixth, the appropriate cutoff values in any study must be determined for each individual PET scanner at each institution.
Conclusion
FDG PET/CT with SUVmax is a useful tool for preoperative evaluation of neck lymph node metastasis in patients with SCC of the pharynx and larynx, especially on the side ipsilateral to the tumor. However, its sensitivity is still insufficient to replace pathologic lymph node staging based on neck dissection.
References
Snow GB, Patel P, Leemans CR, Tiwari R. Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol. 1992;249:187–94.
Castelijins JA, van den Brekel MW. Imaging of lymphadenopathy in the neck. Eur Radiol. 2002;12:727–38.
Ng SH, Yen TC, Liao CT, Chang JT, Chan SC, Ko SF, et al. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: a prospective study of 124 patients with histologic correlation. J Nucl Med. 2005;46:1136–43.
Schwartz DL, Ford E, Rajendran J, Yueh B, Coltrera MD, Virgin J, et al. FDG-PET/CT imaging for preradiotherapy staging of head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:129–36.
Schöder H, Carlson DL, Kraus DH, Stambuk HE, Gönen M, Erdi YE, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med. 2006;47:755–62.
Nabmias C, Carlson ER, Duncan LD, Blodgett TM, Kennedy J, Long MJ, et al. Positron emission tomography/computerized tomography (PET/CT) scanning for preoperative staging of patients with oral/head and neck cancer. J Oral Maxillofac Surg. 2007;65:2524–35.
Pohar S, Brown R, Newman N, Koniarczyk M, Hsu J, Feiglin D. What does PET imaging add to conventional staging of head and neck cancer patients? Int J Radiat Oncol Biol Phys. 2007;68:383–7.
Roh JL, Roh JL, Yeo NK, Kim JS, Lee JH, Cho KJ, et al. Utility of 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol. 2007;43:887–93.
Yamazaki Y, Saitoh M, Notani K, Tei K, Totsuka Y, Takinami S, et al. Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med. 2008;22:177–84.
Krabbe CA, Dijkstra PU, Pruim J, van der Laan BF, van der Wal JE, Gravendeel JP, et al. FDG PET in oral and oropharyngeal cancer. Value for confirmation of N0 neck and detection of occult metastases. Oral Oncol. 2008;44:31–6.
Piao Y, Bold B, Tayier A, Ishida R, Omura K, Okada N, et al. Evaluation of 18F-FDG PET/CT for diagnosing cervical nodal metastases in patients with oral cavity or oropharynx carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:933–8.
Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, Kim JH, et al. CT, MR, US, 18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol. 2009;19:634–42.
Matsubara R, Kawano S, Chikui T, Kiyosue T, Goto Y, Hirano M, et al. Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. Acad Radiol. 2012;19:708–17.
Nguyen A, Luginbuhl A, Cognetti D, Van Abel K, Bar-Ad V, Intenzo C, et al. Effectiveness of PET/CT in the preoperative evaluation of neck disease. Laryngoscope. 2014;124:159–64.
Roh JL, Park JP, Kim JS, Lee JH, Cho KJ, Choi SH, et al. 18F fluorodeoxyglucose PET/CT in head and neck squamous cell carcinoma with negative neck palpation findings: a prospective study. Radiology. 2014;271:153–61.
Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000;174:837–44.
Sakai O, Curtin HD, Romo LV, Som PM. Lymph node pathology: benign proliferative, lymphoma, and metastatic disease. Radiol Clin North Am. 2000;38:979–98.
Ferlito A, Rinaldo A, Silver CE, Gourin CG, Shah JP, Clayman GL, et al. Elective and therapeutic selective neck dissection. Oral Oncol. 2006;42:14–25.
Sun R, Tang X, Yang Y, Zhang C. 18FDG-PET/CT for the detection of regional nodal metastasis in patients with head and neck cancer: a meta-analysis. Oral Oncol. 2015;51:314–20.
Kim SY, Kim JS, Doo H, Lee H, Lee JH, Cho KJ, et al. Combined [18F]fluorodeoxyglucose positron emission tomography and computed tomography for detecting contralateral neck metastases in patients with head and neck squamous cell carcinoma. Oral Oncol. 2011;47:376–80.
Kastrinidis N, Kuhn FP, Hany TF, Ahmad N, Huber GF, Haerle SK. 18F-FDG-PET/CT for the assessment of the contralateral neck in patients with head and neck squamous cell carcinoma. Laryngoscope. 2013;123:1210–5.
Joo YH, Yoo IR, Cho KJ, Park JO, Nam IC, Kim CS, et al. The value of preoperative 18F-FDG PET/CT for the assessing contralateral neck in head and neck cancer patients with unilateral node metastasis (N1-3). Clin Otolaryngol. 2014;39:338–44.
Takamochi K, Yoshida J, Murakami K, Niho S, Ishii G, Nishimura M, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer. 2005;47:235–42.
Stoeckli SJ, Pfaltz M, Steinert H, Schmid S. Histopathological features of occult metastasis detected by sentinel lymph node biopsy in oral and oropharyngeal squamous cell carcinoma. Laryngoscope. 2002;112:111–5.
Bundschuh RA, Essler M, Dinges J, Berchtenbreiter C, Mariss J, Martínez-Möller A, et al. Semiautomatic algorithm for lymph node analysis corrected for partial volume effects in combined positron emission tomography-computed tomography. Mol Imaging. 2010;9:319–28.
Acknowledgments
We thank Takashi Okunaga RT and Kazuhiro Kubo RT (Radiology Division, Kobe University Hospital, Kobe, Japan) for their excellent technical assistance and generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no financial support or relationship that may pose conflict of interest.
About this article
Cite this article
Suenaga, Y., Kitajima, K., Kanda, T. et al. [18F]-FDG PET/CT imaging for detection of nodal metastases in patients with squamous cell carcinoma of the pharynx and larynx: comparison with CT. Jpn J Radiol 34, 203–210 (2016). https://doi.org/10.1007/s11604-015-0510-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0510-6