Abstract
Background
Adenomyosis is a benign disorder characterized by the presence of ectopic endometrial glands and stroma within the myometrium. The main clinical manifestations of adenomyosis are dysmenorrhea, menorrhagia, and infertility, which affect patients’ quality of life. Recently, with advancements in imaging techniques, magnetic resonance imaging, and ultrasonography have become the main diagnostic tools for adenomyosis. In addition to the diagnosis and differential diagnosis of adenomyosis, ultrasonography can also be used to evaluate the severity of adenomyosis. The emergence of new techniques, such as elastography and contrast-enhanced ultrasonography (CEUS), has significantly improved the accuracy of ultrasound-based diagnosis of adenomyosis. These two imaging tools can also be used for the differential diagnosis of adenomyosis and the evaluation of treatment efficacy after medication or ablation procedure.
Objective
we review the efficacy of ultrasonography as a diagnostic tool for adenomyosis. We also aim to introduce the potential of ultrasound imaging in the evaluation of the severity of this disease, as well as the application of elastography and contrast-enhanced ultrasonography (CEUS) in its diagnosis.
Results and Conclusion
Our findings reveal the potential value of ultrasonography combined with elastography and/or CEUS as medication guidance and efficacy evaluation tools in the long-term management of adenomyosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Early diagnosis and intervention for adenomyosis can help improve patients’ quality of life. The use of elastography and contrast-enhanced ultrasonography, as well as ultrasound-based staging of the se-verity of adenomyosis, can help diagnose and treat adenomyosis at an early stage and individualize treatment. |
Introduction
Adenomyosis is a common benign condition characterized by the infiltration of ectopic endometrial glands and stroma within the uterus. The prevalence of adenomyosis, based on histopathological diagnoses, ranges from 5 to 70% [1, 2]. Although some patients may be asymptomatic, most experience dysmenorrhea, menorrhagia, and chronic pelvic pain, which adversely affect patients’ quality of life and psychological health. Additionally, the presence of adenomyosis is discovered at a high frequency in patients who consult for fertility problems [3]. Increased infertility and miscarriage rates have been found in women with adenomyosis, compared with those of women without adenomyosis [4, 5]. In addition, although some patients show no early symptoms, the disease still progresses according to the repeated tissue injury and repair (ReTIAR) theory [6, 7], and progressive dysmenorrhea, increased menstrual volume, infertility, and other manifestations may appear later. Therefore, it is crucial to diagnose adenomyosis as early as possible to improve patients’ quality of life. Early diagnosis of adenomyosis and classification of its severity can also help to individualize treatment and manage the disease.
Although histopathological diagnosis remains the gold standard for the identification of adenomyosis, the development of imaging tools has made non-invasive diagnosis more accurate, allowing better adenomyosis detection [8]. Compared with magnetic resonance imaging (MRI), ultrasonography is less expensive. It also can offer dynamic imaging and real-time observation. Moreover, ultrasonography is easy to implement and replicate. There are also systematic, standardized ultrasound reports based on DEGUM, ÖGUM, and SGUM recommendations [9]. As a result, ultrasonography has become the first-line imaging tool in the diagnosis of adenomyosis [6, 8]. In addition to diagnosis, ultrasonography can also be used to monitor and evaluate changes in adenomyotic lesions during hormonal therapies. In this paper, we review the efficacy of ultrasonography as a diagnostic tool for adenomyosis. We also aim to discuss the potential of ultrasonographic imaging in the evaluation of the severity of this disease, as well as the application of elastography and contrast-enhanced ultrasonography (CEUS) in its diagnosis.
Diagnostic properties of ultrasonography in adenomyosis
In 2015, Van et al. issued the Morphological Uterus Sonographic Assessment (MUSA), a consensus statement on terms, definitions, and measurements, to facilitate the description and reporting of myometrial sonographic features [10]. Adenomyosis is usually defined by the occurrence of one or more of the following sonographic features based on the MUSA criteria: a globally enlarged uterus, asymmetrical thickness of the uterine wall, subendometrial lines and buds, hyperechogenic islands within the myometrium, myometrial cysts, presence of fan-shaped shadowing, an irregular or interrupted junctional zone (JZ), and/or translesional vascularity [10, 11].
Although transabdominal ultrasonography (TAUS) may be useful when the vaginal route is not accessible or in cases of a considerably enlarged uterus, its value is limited [12]. In contrast, transvaginal ultrasonography (TVUS) provides an optimal view of the uterus using two-dimensional (2D) and three-dimensional (3D) settings, together with a Power/Color Doppler, and has a higher sensitivity than TAUS [13, 14].
With the advancement of ultrasonographic techniques and the uniformity of sonographic terms in recent decades, the diagnostic accuracy of TVUS has improved, being comparable to that of MRI [15]. Moreover, owing to its inexpensiveness, ease of use, ability to perform dynamic examination, and good inter-rater agreement with 2D [8, 16], TVUS has become the first-line imaging technique. Although traditional 2D TVUS was considered unreliable in the evaluation of the JZ, compared with MRI, the appearance of 3D ultrasonography made it valuable to evaluate the morphology and thickness of the JZ [17]. However, a recent meta-analysis suggested that there was no improvement in overall accuracy in 3D TVUS, compared with 2D TVUS, for the diagnosis of adenomyosis, although 3D TVUS eased the evaluation of JZ [18].
In addition to studying the pooled accuracy of ultrasonographic diagnosis for adenomyosis, some studies have also compared the single sensitivity and specificity of different sonographic features. Andres et al. [18] published a systematic review of the accuracy of 2D and 3D TVUS, which included eight articles published up to the last few decades. They reported that the pooled sensitivity and specificity of 2D TVUS for the diagnosis of adenomyosis for all combined imaging features were 83.8% and 63.9%, respectively. For a single imaging feature, the heterogeneous myometrium had the highest sensitivity (86.0%), whereas the globular uterus had the highest specificity (78.1%). For 3D TVUS, the pooled sensitivity and specificity for all combined imaging characteristics were 88.9% and 56.0%, respectively. Poor definition of the JZ showed the highest pooled sensitivity (86%) and specificity (56.0%) for the diagnosis of adenomyosis using 3D TVUS.
A prospective study, which was aimed at determining the most valuable sonographic features in the diagnosis of adenomyosis [19], stated that a regularly enlarged uterus with a globular appearance, subendometrial echogenic linear striations, and myometrial cysts showed the highest accuracy as indicators of adenomyosis. Of those, the subendometrial echogenic linear striations were considered the most specific feature to diagnose this disease.
Liu et al. [15] also found that the most used imaging characteristics were asymmetrical thickening, myometrial cysts, and echogenic subendometrial lines and buds. The subendometrial lines and buds was the best criterion, with an area under the receiver-operating characteristic curve value of 0.83 (95% confidence interval, 0.79–0.86).
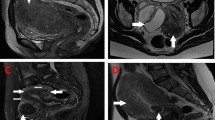
In a previous study [20], poor inter- and intra-rater agreement when using the MUSA criteria for adenomyosis diagnosis was found both among highly experienced and moderately experienced raters. The results showed that correct identification of ill-defined lesions was difficult, even when using MUSA features of adenomyosis. As a result, Van et al. [21] aimed to update the MUSA definitions of adenomyosis, if necessary, and to reach a consensus on the updated definitions, easing the clarification of these features. This study further determined the necessity to distinguish between direct features, indicating the presence of ectopic endometrium and stroma inside the myometrium, and indirect features, representing secondary changes in the presence of endometrial tissue in the myometrium. Myometrial cysts, hyperechogenic islands, and subendometrial lines and buds were unanimously classified as direct features of adenomyosis, while all others were classified as indirect features (Fig. 1).
Association between sonographic features and adenomyotic symptoms
In recent years, ultrasonography has been used not only to diagnose diseases but also to assess disease severity and guide treatment. It has been reported [22] that TVUS has good consistency in predicting endometriosis severity, especially in higher disease stages assessed by the revised American Society for Reproductive Medicine. TVUS combined with Enzian classification can also accurately evaluate the severity of endometriosis. Further, some common findings in endometriosis were found to be associated with gastrointestinal symptoms [23]. This implies that TVUS can assess the complexity of the surgery and the risk of postoperative complications. Several articles have also attempted to study the correlation between TVUS and the severity and symptoms of adenomyosis. Studies have found that different adenomyosis phenotypes may manifest different clinical symptoms [24, 25]. One of those studies [25] reported that patients with diffuse adenomyosis were more likely to be older, infertile, and presenting with abnormal uterine bleeding (AUB) than patients with focal or no adenomyosis. In this study, a more irregular, unclear, and interrupted JZ was observed in patients with AUB. A study on adenomyosis in adolescents [26] also found that dysmenorrhea was mainly associated with adenomyosis of the outer myometrium. Moreover, heavy menstrual bleeding was associated with diffuse adenomyosis mostly in the outer myometrium, and dyspareunia was linked to diffuse adenomyosis, including both the inner and outer myometrium. Myometrial hyperechoic areas, uterine wall asymmetry, and intramyometrial cystic areas were the most common sonographic features of adenomyosis in adolescents with dysmenorrhea.
The association between sonographic features and adenomyotic symptoms, such as dysmenorrhea, menorrhagia, and infertility, has been a hot topic of discussion in the diagnosis of adenomyosis using TVUS. Naftalin et al. [27] assessed adenomyosis severity based on the number of imaging features. They reported an increase in adenomyosis severity linked to the increase in the number of imaging features involved. They found that the severity of adenomyosis was positively associated with the numerical rating scale score and the amount of menstrual loss estimated by the pictorial blood loss analysis chart (PBCA) score, although no significant association was found between ultrasound-diagnosed adenomyosis and menorrhagia. These results were consistent with those of their early research [28]. Pinzauti et al. [29] also found a significant relationship between the number of 2D TVUS diagnosis features of diffuse adenomyosis and the visual analog scale score for dysmenorrhea and the PBCA score for menstrual loss. Notably, all sonographic features of adenomyosis can be characterized as direct or indirect, as described in the study by Van et al. [21], which suggests that different sonographic features may reflect different levels of adenomyosis severity. As a result, there may be some limitations to this approach. Some patients may have severe symptoms but lack sonographic features of adenomyosis, whereas others may have the opposite.
Recently, instead of simply evaluating the severity of adenomyosis based on the number of sonographic features, experts have made efforts to develop new systems for the classification and staging of adenomyosis. Lazzeri et al. [30] developed a new ultrasonographic mapping system to define the type and extent of adenomyosis. In their system, adenomyosis was divided into diffuse, focal, and adenomyomas. Furthermore, the extension of the lesions and the thickness of the JZ were measured. Each type and score of adenomyosis was evaluated by the system and divided into four subgroups. Finally, the sum of the single degrees of each type of adenomyosis represented the severity of the adenomyosis. Good inter-observer agreement was found when using the system. They further correlated the type and grade of adenomyosis, scored through the system, and the severity of symptoms [31]. The study found that heavier menstrual bleeding was correlated with more severe diffuse adenomyosis, whereas focal adenomyosis was associated with a higher infertility rate. Women with focal adenomyosis and affected JZs had a higher miscarriage rate of at least one than those with diffuse adenomyosis. Moreover, they found a tendency for a higher percentage of miscarriages in women with severe adenomyosis than that in those with mild adenomyosis. Tamura et al. [32] also found higher rates of miscarriage and cervical incompetency in patients with an adenomyosis size of > 60 mm.
Value of elastography in adenomyosis diagnosis
Traditional TVUS, both 2D and 3D, still has some limitations in the diagnosis of adenomyosis, mainly derived from the inherent subjectivity of most of the sonographic features. Elastography, as a new sonography pattern, can objectively reflect tissue stiffness. Elastography can be roughly divided into two different forms: stain imaging and shear-wave imaging [33]. Stain-imaging measures formation by applying pressure to the probe, whereas shear-wave imaging records the propagation speed of the shear wave after excitation. Currently, this technique has proven useful in assessing the extent of fibrosis and distinguishing benign and malignant diseases in the liver, breast, and other organs [34]. Applications of elastography in improving the diagnosis of deep endometriosis have also been proved [35]. In elastography, tissue stiffness is usually displayed by an image encoded by four colors (red, yellow, green, and blue) aside from the traditional B-mode image. Red and blue represent the softest and the hardest tissues, respectively, while yellow and green are attributed to the tissue with moderate degree of stiffness [6, 36]. In studies on elastography diagnosis of adenomyosis, the lesional stiffness was semi-quantified mainly by the ratio of elasticity between the lesion and the adjacent myometrium or between the lesion and the endometrium [36, 37]. A combination between traditional ultrasonography and elastography can significantly improve the sensitivity and specificity of disease diagnosis, compared with traditional ultrasonography alone [38, 39].
Owing to its characteristics, elastography is typically used in the differential diagnosis of adenomyosis and uterine fibroids. Several studies reported that adenomyosis was stiffer than the uterine fibroids, and both were stiffer than the normal myometrium (Fig. 2). The fibroids showed a clear pseudocapsule with red coloration, while adenomyosis had no clear boundary with the surrounding myometrium [6, 36, 39, 40]. In contrast, some studies found that the stiffness of adenomyosis was lower than that of fibroids, and even lower than that of normal myometrium [41, 42]. The controversy regarding the stiffness of adenomyosis may be attributed to the following reasons: (1) the stain ratio measured was affected by probe pressure, which may be uniform; (2) the selection of a region of interest (ROI) was subjective; (3) adenomyosis lesions usually do not have defined boundary shifts on ultrasonography or elastography; (4) the reference ratio values were obtained by different approaches [37, 43].
Elastosonographic image of adenomyosis. In the figure, red represents the hardest tissue and blue represents the softest tissue, which is contrary to the elastography color settings in most articles. This may be because the image was obtained using ultrasonic machines made in China with slightly different parameter Settings from other ultrasonic machines. “妇科病变1应变比A” meant the stain percentage of gynecological lesion 1 (adenomyotic lesion), “妇科病变2应变比” meant the stain percentage of gynecological lesion 1(the adjacent normal myometrium). “妇科病变1应变比B/A” represented the stiffness of adenomyotic lesion calculated by the ratio of stain percentage between the lesion and the adjacent myometrium
Recently, an increasing number of studies have found that the extent of fibrosis, reflected by lesion stiffness with the use of elastography, correlated positively with dysmenorrhea [6, 44, 45]. In addition, a positive correlation between menorrhagia and fibrosis severity was also reported [46]. These studies have demonstrated the great potential of elastography for the clinical diagnosis of adenomyosis and evaluation of the severity of its symptoms. A negative correlation between progesterone receptor expression and lesion stiffness was also reported in a previous study [6]. These findings suggest that elastography may be useful in guiding medical therapy and evaluating its efficacy in adenomyosis. A prospective study [47] that used four colors and elasticity scores (ESs) (Table 1) to assess lesional stiffness found that the lesional ESs of patients with adenomyosis increased after GnRHa treatment. Chiara et al. [48] studied the value of real-time elastography with a transvaginal approach, assessing the response to MRgFUS treatment of uterine fibroid. They found a reduction in the stain ratio (SR, ROI lesions/ROI normal myometrium) for fibroids after treatment, compared with that of those before treatment. In particular, a significant decrease in SR was found in the patient group with a non-perfusion volume of > 70%.
However, it is important to note that elastography still has some limitations: (1) strain elastography cannot reflect the absolute tissue stiffness because of its operator-dependent property, and (2) it remains unknown whether elastography may help less-skilled sonographers in the diagnosis and differential diagnosis of diseases [35].
Value of contrast-enhanced ultrasonography in adenomyosis diagnosis
CEUS is an emerging non-invasive imaging technique for the real-time evaluation of tissue perfusion with qualitative and quantitative analyses. The imaging technique uses microbubble contrast agents, which are safer than iodine contrast [49, 50]. Compared with enhanced MRI, CEUS has the following advantages: (1) it is cheaper and easier to perform; (2) more than one contrast imaging can be acquired during an examination to obtain better imaging for the ROI; and (3) it allows dynamic real-time imaging with high spatial and temporal capabilities [49, 51, 52]. The main qualitative parameters of CEUS include enhancement level, enhancement pattern, enhancement time of the lesion, lesion boundary after enhancement, and contrast agent distribution. Quantitative parameters were acquired from the time–intensity curves, including arrival time, time to peak, peak intensity, and mean transit time [50]. CEUS is better at evaluating microvascular perfusion than Color Doppler ultrasonography, which means that it can better reflect the distribution of blood vessels in and around the lesions and has good value in distinguishing benign from malignant diseases [50].
CEUS can also be used in the differential diagnosis of benign and malignant diseases in the liver, thyroid, ovaries, and other organs [53,54,55,56]. Several studies have also investigated the value of CEUS in evaluating the efficacy of ablation in the treatment of uterine fibroids [57, 58]. Regarding the application of CEUS in adenomyosis, several studies have explored its value in the differential diagnosis of adenomyosis and the evaluation of the efficacy of its treatment. A prospective study found statistically significant differences in the quantitative and qualitative parameters of CEUS between adenomyosis and uterine fibroids [50]. Four independent risk factors for focal adenomyosis were identified, and a CEUS model was established to distinguish focal adenomyosis from uterine fibroids. Good sensitivity and specificity were confirmed by the CEUS model in this study (derivation cohort, 98.33 and 70.00%, respectively; validation cohort, both 85.71%). Some studies reported that CEUS can also accurately evaluate the efficacy of microwave ablation or high-intensity focused ultrasound for adenomyosis by assessing the ablation rate of localized adenomyosis [59, 60]. The ablation rate measured by CEUS was essentially consistent with that measured by dynamic contrast-enhanced MRI (90.90% ± 6.61% vs. 90.88% ± 6.32%, respectively) [60].
Although CEUS has been widely used in tumor diagnosis or follow-up after local treatment in recent years [61], its application in adenomyosis has been less studied. Therefore, further studies are needed to evaluate the value and safety of CEUS in the diagnosis and treatment efficacy of adenomyosis.
Summary
Adenomyosis is characterized by the progressive worsening of secondary dysmenorrhea, menorrhagia, and infertility, which seriously affect patients’ life and mental states. Some patients with adenomyosis may have significant dysmenorrhea or menorrhagia without typical imaging findings, leading to a delayed diagnosis. Early diagnosis and intervention in adenomyosis, as well as selection of appropriate treatment options, can help improve the quality of life of these patients. Ultrasonographic imaging techniques, which have similar specificities and sensitivities to MRI, have become the first-line diagnostic approach due to their prices and availabilities. The severity of adenomyosis and associated symptoms can also be evaluated using 2D and 3D ultrasonography. Elastography and CEUS, which are emerging imaging tools in gynecology and obstetrics, can greatly improve the specificity and sensitivity of diagnosis. They can also ease the differential diagnosis of adenomyosis by ultrasonography and the evaluation of the efficacy of conservative treatments for this disease. These results demonstrate the potential value of ultrasonography combined with elastography and/or CEUS as medication guidance and efficacy evaluation tools in the long-term management of adenomyosis. However, more studies with larger sample sizes are still needed to confirm the value of elastography and CEUS in the diagnosis and long-term management of adenomyosis. A model combining clinical manifestations, laboratory findings, conventional ultrasonography and elastography findings, and CEUS may be useful to predict the occurrence and severity of adenomyosis and, therefore, facilitate its early diagnosis, medication guidance, and efficacy assessment.
Data Availability
Data availability is not applicable to this article as no new data were created or analyzed in this study
References
Van den Bosch T, Van Schoubroeck D (2018) Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract Res Clin Obstet Gynaecol 51:16–24. https://doi.org/10.1016/j.bpobgyn.2018.01.013
Cunningham RK, Horrow MM, Smith RJ, Springer J (2018) Adenomyosis: a sonographic diagnosis. Radiographics 38(5):1576–1589. https://doi.org/10.1148/rg.2018180080
Harada T, Khine YM, Kaponis A, Nikellis T, Decavalas G, Taniguchi F (2016) The impact of adenomyosis on women’s fertility. Obstet Gynecol Surv 71(9):557–568. https://doi.org/10.1097/ogx.0000000000000346
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E (2014) Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 29(5):964–977. https://doi.org/10.1093/humrep/deu041
Younes G, Tulandi T (2017) Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril 108(3):483-490.e3. https://doi.org/10.1016/j.fertnstert.2017.06.025
Liu X, Ding D, Ren Y, Guo SW (2018) Transvaginal elastosonography as an imaging technique for diagnosing adenomyosis. Reprod Sci 25(4):498–514. https://doi.org/10.1177/1933719117750752
Guo SW (2022) Cracking the enigma of adenomyosis: an update on its pathogenesis and pathophysiology. Reproduction 164(5):R101–R121. https://doi.org/10.1530/rep-22-0224
Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS et al (2020) Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update 26(3):392–411. https://doi.org/10.1093/humupd/dmz049
Grab D, Merz E, Eichhorn KH, Tutschek B, Kagan KO, Heling KS et al (2022) Basic gynecologic ultrasound examination (Level I): DEGUM, ÖGUM, and SGUM recommendations. Ultraschall Med. https://doi.org/10.1055/a-1851-5157.10.1055/a-1851-515
Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A et al (2015) Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 46(3):284–298. https://doi.org/10.1002/uog.14806
Van den Bosch T, de Bruijn AM, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L et al (2019) Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol 53(5):576–582. https://doi.org/10.1002/uog.19096
Bazot M, Daraï E, Rouger J, Detchev R, Cortez A, Uzan S (2002) Limitations of transvaginal sonography for the diagnosis of adenomyosis, with histopathological correlation. Ultrasound Obstet Gynecol 20(6):605–611. https://doi.org/10.1046/j.1469-0705.2002.00852.x
Levy G, Dehaene A, Laurent N, Lernout M, Collinet P, Lucot JP et al (2013) An update on adenomyosis. Diagn Interv Imaging 94(1):3–25. https://doi.org/10.1016/j.diii.2012.10.012
Dueholm M (2006) Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best practice and research. Best Pract Res Clin Obstet Gynaecol 20(4):569–582. https://doi.org/10.1016/j.bpobgyn.2006.01.005
Liu L, Li W, Leonardi M, Condous G, Da Silva CF, Mol BW et al (2021) Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med 40(11):2289–2306. https://doi.org/10.1002/jum.15635
Rasmussen CK, Hansen ES, Dueholm M (2019) Inter-rater agreement in the diagnosis of adenomyosis by 2- and 3-dimensional transvaginal ultrasonography. J Ultrasound Med 38(3):657–666. https://doi.org/10.1002/jum.14735
Exacoustos C, Brienza L, Di Giovanni A, Szabolcs B, Romanini ME, Zupi E et al (2011) Adenomyosis: three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol 37(4):471–479. https://doi.org/10.1002/uog.8900
Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM (2018) Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol 25(2):257–264. https://doi.org/10.1016/j.jmig.2017.08.653
Kepkep K, Tuncay YA, Göynümer G, Tutal E (2007) Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound Obstet Gynecol 30(3):341–345. https://doi.org/10.1002/uog.3985
Rasmussen CK, Van den Bosch T, Exacoustos C, Manegold-Brauer G, Benacerraf BR, Froyman W et al (2019) Intra- and inter-rater agreement describing myometrial lesions using morphologic uterus sonographic assessment: a pilot study. J Ultrasound Med 38(10):2673–2683. https://doi.org/10.1002/jum.14971
Harmsen MJ, Van den Bosch T, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L et al (2022) Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: results of modified Delphi procedure. Ultrasound Obstet Gynecol 60(1):118–131. https://doi.org/10.1002/uog.24786
Keckstein J, Hoopmann M, Merz E, Grab D, Weichert J, Helmy-Bader S et al (2023) Expert opinion on the use of transvaginal sonography for presurgical staging and classification of endometriosis. Arch Gynecol Obstet 307(1):5–19. https://doi.org/10.1007/s00404-022-06766-z
Cohen Ben-Meir L, Soriano D, Zajicek M, Yulzari V, Bouaziz J, Beer-Gabel M et al (2022) The association between gastrointestinal Symptoms and Transvaginal Ultrasound Findings in women referred for endometriosis evaluation: a prospective pilot study. Ultraschall Med 43(5):e81–e89. https://doi.org/10.1055/a-1300-188725
Bourdon M, Oliveira J, Marcellin L, Santulli P, Bordonne C, Maitrot Mantelet L et al (2021) Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum Reprod 36(2):349–357. https://doi.org/10.1093/humrep/deaa307
Marques ALS, Andres MP, Mattos LA, Gonçalves MO, Baracat EC, Abrão MS (2021) Association of 2D and 3D transvaginal ultrasound findings with adenomyosis in symptomatic women of reproductive age: a prospective study. Clinics (Sao Paulo) 76:e2981. https://doi.org/10.6061/clinics/2021/e2981
Exacoustos C, Lazzeri L, Martire FG, Russo C, Martone S, Centini G et al (2022) Ultrasound findings of Adenomyosis in Adolescents: type and grade of the disease. J Minim Invasive Gynecol 29(2):291-299.e1. https://doi.org/10.1016/j.jmig.2021.08.023
Naftalin J, Hoo W, Nunes N, Holland T, Mavrelos D, Jurkovic D (2016) Association between ultrasound features of adenomyosis and severity of menstrual pain. Ultrasound Obstet Gynecol 47(6):779–783. https://doi.org/10.1002/uog.15798
Naftalin J, Hoo W, Pateman K, Mavrelos D, Foo X, Jurkovic D (2014) Is adenomyosis associated with menorrhagia? Hum Reprod 29(3):473–479. https://doi.org/10.1093/humrep/det451
Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S et al (2015) Transvaginal sonographic features of diffuse adenomyosis in 18–30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol 46(6):730–736. https://doi.org/10.1002/uog.14834
Lazzeri L, Morosetti G, Centini G, Monti G, Zupi E, Piccione E et al (2018) A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril 110(6):1154-1161.e3. https://doi.org/10.1016/j.fertnstert.2018.06.031
Exacoustos C, Morosetti G, Conway F, Camilli S, Martire FG, Lazzeri L et al (2020) New sonographic classification of Adenomyosis: do type and degree of Adenomyosis correlate to severity of symptoms? J Minim Invasive Gynecol 27(6):1308–1315. https://doi.org/10.1016/j.jmig.2019.09.788
Tamura H, Kishi H, Kitade M, Asai-Sato M, Tanaka A, Murakami T et al (2017) Complications and outcomes of pregnant women with adenomyosis in Japan. Reprod Med Biol 16(4):330–336. https://doi.org/10.1002/rmb2.1205034
Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol 41(5):1126–1147. https://doi.org/10.1016/j.ultrasmedbio.2015.03.009
Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH et al (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography Part 2: clinical applications. Ultraschall Med 34(3):238–253. https://doi.org/10.1055/s-0033-1335375
Xholli A, Londero APP, Cavalli E, Scovazzi U, Ferraro MF, Vacca I et al (2023) The benefit of transvaginal elastography in detecting deep endometriosis: a feasibility study. Ultraschall Med. https://doi.org/10.1055/a-2028-8214.10.1055/a-2028-821437
Săsăran V, Turdean S, Gliga M, Ilyes L, Grama O, Muntean M et al (2021) Value of Strain-ratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med 11(8):824. https://doi.org/10.3390/jpm11080824
Görgülü FF, Okçu NT (2021) Which imaging method is better for the differentiation of adenomyosis and uterine fibroids? J Gynecol Obstet Hum Reprod 50(5):102002. https://doi.org/10.1016/j.jogoh.2020.102002
Wei Q, Yan YJ, Wu GG, Ye XR, Jiang F, Liu J et al (2021) Added value of a new strain elastography technique in conventional ultrasound for the diagnosis of breast masses: a prospective multicenter study. Front Oncol 11:779612. https://doi.org/10.3389/fonc.2021.779612
Săsăran V, Turdean S, Mărginean C, Gliga M, Ilyes L, Grama O et al (2022) Transvaginal ultrasound combined with strain-ratio elastography for the concomitant diagnosis of uterine fibroids and adenomyosis: a pilot study. J Clin Med 11(13):3757. https://doi.org/10.3390/jcm11133757
Acar S, Millar E, Mitkova M, Mitkov V (2016) Value of ultrasound shear wave elastography in the diagnosis of adenomyosis. Ultrasound 24(4):205–213. https://doi.org/10.1177/1742271X16673677
Frank ML, Schäfer SD, Möllers M, Falkenberg MK, Braun J, Möllmann U et al (2016) Importance of transvaginal elastography in the diagnosis of uterine fibroids and adenomyosis. Ultraschall Med 37(4):373–378. https://doi.org/10.1055/s-0035-1553266
Tessarolo M, Bonino L, Camanni M, Deltetto F (2011) Elastosonography: a possible new tool for diagnosis of adenomyosis? Eur Radiol 21(7):1546–1552. https://doi.org/10.1007/s00330-011-2064-z
Wang XL, Lin S, Lyu GR (2012) Advances in the clinical application of ultrasound elastography in uterine imaging. Insights Imag 13(1):141. https://doi.org/10.1186/s13244-022-01274-9
Wang S, Li B, Duan H, Wang Y, Shen X, Dong Q (2021) Abnormal expression of connective tissue growth factor and its correlation with fibrogenesis in adenomyosis. Reprod Biomed Online 42(3):651–660. https://doi.org/10.1016/j.rbmo.2020.11.002
Yang B, Gu N, Shi S, Zhang C, Chen L, Ouyang J et al (2021) Immunoreactivity of plasminogen activator inhibitor 1 and Its correlation with dysmenorrhea and lesional fibrosis in adenomyosis. Reprod Sci 28(8):2378–2386. https://doi.org/10.1007/s43032-021-00513-6
Huang Q, Liu X, Critchley H, Fu Z, Guo SW (2022) How does the extent of fibrosis in adenomyosis lesions contribute to heavy menstrual bleeding? Reprod Med Biol 21(1):e12442. https://doi.org/10.1002/rmb2.12442
Xie M, Yu H, Zhang X, Wang W, Ren Y (2019) Elasticity of adenomyosis is increased after GnRHa therapy and is associated with spontaneous pregnancy in infertile patents. J Gynecol Obstet Hum Reprod 48(10):849–853. https://doi.org/10.1016/j.jogoh.2019.05.003
Marigliano C, Panzironi G, Molisso L, Pizzuto A, Ciolina F, Napoli A et al (2016) First experience of real-time elastography with transvaginal approach in assessing response to MRgFUS treatment of uterine fibroids. Radiol Med 121(12):926–934. https://doi.org/10.1007/s11547-016-0679-5
Chhabra P, Daugherty R, LeNoir AM, Grilli C, Makai G, Patel N et al (2021) Comparison of contrast-enhanced ultrasound versus magnetic resonance imaging in the detection and characterization of Uterine Leiomyomas. J Ultrasound Med 40(6):1147–1153. https://doi.org/10.1002/jum.15495
Zhang YQ, Chen JH, Zhu TT, Zhao AX, Zhuang LT, Lu CY et al (2022) Applying contrast-enhanced ultrasound model to distinguish atypical focal adenomyosis from uterine leiomyomas. Ann Transl Med 10(20):1108
Durot I, Wilson SR, Willmann JK (2018) Contrast-enhanced ultrasound of malignant liver lesions. Abdom Radiol (NY) 43(4):819–847. https://doi.org/10.1007/s00261-017-1360-8
Liu X, Jang HJ, Khalili K, Kim TK, Atri M (2018) Successful Integration of contrast-enhanced US into routine abdominal imaging. Radiographics 38(5):1454–1477. https://doi.org/10.1148/rg.2018170152
Pang T, Huang L, Deng Y, Wang T, Chen S, Gong X et al (2017) Logistic regression analysis of conventional ultrasonography, strain elastosonography, and contrast-enhanced ultrasound characteristics for the differentiation of benign and malignant thyroid nodules. PLoS ONE 12(12):e0188987. https://doi.org/10.1371/journal.pone.0188987
Green RW, Epstein E (2020) Dynamic contrast-enhanced ultrasound improves diagnostic performance in endometrial cancer staging. Ultrasound Obstet Gynecol 56(1):96–105. https://doi.org/10.1002/uog.21885
Dietrich CF (2019) Contrast-Enhanced Ultrasound of Benign Focal Liver Lesions. Ultraschall Med 40(1):12–29. https://doi.org/10.1055/a-0668-5746
Ma X, Zhao Y, Zhang B, Ling W, Zhuo H, Jia H et al (2015) Contrast-enhanced ultrasound for differential diagnosis of malignant and benign ovarian tumors: systematic review and meta-analysis. Ultrasound Obstet Gynecol 46(3):277–283. https://doi.org/10.1002/uog.14800
Peng S, Xiong Y, Li K, He M, Deng Y, Chen L et al (2012) Clinical utility of a microbubble-enhancing contrast (“SonoVue”) in treatment of uterine fibroids with high intensity focused ultrasound: a retrospective study. Eur J Radiol 81(12):3832–3838. https://doi.org/10.1016/j.ejrad.2012.04.030
Jiang N, Xie B, Zhang X, He M, Li K, Bai J et al (2014) Enhancing ablation effects of a microbubble-enhancing contrast agent (“SonoVue”) in the treatment of uterine fibroids with high-intensity focused ultrasound: a randomized controlled trial. Cardiovasc Intervent Radiol 37(5):1321–1328. https://doi.org/10.1007/s00270-013-0803-z
Wang W, Wang Y, Tang J (2009) Safety and efficacy of high intensity focused ultrasound ablation therapy for adenomyosis. Acad Radiol 16(11):1416–1423. https://doi.org/10.1016/j.acra.2009.06.005
Xu C, Tang Y, Zhao Y, Li Y, Feng Q (2020) Use of contrast-enhanced ultrasound in evaluating the efficacy and application value of microwave ablation for adenomyosis. J Cancer Res Ther 16(2):365–371. https://doi.org/10.4103/jcrt.jcrt_769_18
Cheng CQ, Zhang RT, Xiong Y, Chen L, Wang J, Huang GH et al (2015) Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases: retrospective analysis of contrast safety. Medicine (Baltimore) 94(16):e729. https://doi.org/10.1097/MD.0000000000000729
Funding
This work was funded by the National Key R&D Program of China (Grant No. 2022YFC2704002), National Natural Science Foundation of China (Grant No. 82071621), and Major Basic Research of Natural Science Foundation of Shandong Province (Grant No. ZR2021ZD34).
Author information
Authors and Affiliations
Contributions
RQ: manuscript writing and literature review; YM: manuscript writing and editing; GW: manuscript writing and editing, and project development.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, Q., Yuan, M. & Wang, G. Role of ultrasonography in the evaluation of disease severity and treatment efficacy in adenomyosis. Arch Gynecol Obstet 309, 363–371 (2024). https://doi.org/10.1007/s00404-023-07034-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07034-4