Abstract
Introduction
To investigate whether microsatellite instability (MSI) is an important prognostic biomarker for endometrioid endometrial cancer (EEC).
Methods
The PubMed, EMBASE, and the Cochrane Cooperative Library databases were searched from inception to July 2021. Overall survival, disease-free survival, progression-free survival, EEC-specific survival, recurrence-free survival, and the recurrence rate were pooled to analyze the correlation between MSI and EEC. In addition, Egger’s regression analysis and Begg’s test were used to detect publication bias.
Results
17 studies met the inclusion criteria and were included in our meta-analysis with a sample size of 4723, and the included patients with endometrioid cancer (EC) all were EEC. The pooled hazard ratios (HR) in patients with EEC showed that MSI was significantly associated with shorter overall survival [HR = 1.37, 95% confidence interval (CI) (1.00–1.86), p = 0.048, I2 = 60.6%], shorter disease-free survival [HR = 1.99, 95% CI (1.31–3.01), p = 0.000, I2 = 67.2%], shorter EEC-specific survival [HR = 2.07, 95% CI (1.35–3.18), p = 0.001, I2 = 31.6%] and a higher recurrence rate [Odds ratios (OR) = 2.72, 95% CI (1.56–4.76), p = 0.000, I2 = 0.0%]. In the early-stage EEC subgroup, MSI was significantly associated with shorter overall survival [HR = 1.47, 95% CI (1.11–1.95), p = 0.07], shorter disease-free survival [HR = 4.17, 95% CI (2.37–7.41), p = 0.000], and shorter progression-free survival [HR = 2.41, 95% CI (1.05–5.54), p = 0.039]. No significant heterogeneity was observed in overall survival (I2 = 20.9%), disease-free survival (I2 = 0.0%), or progression-free survival (I2 = 0.0%) in patients with early-stage EEC. Meanwhile, publication bias was not observed, and the p-value for Egger’s test of overall survival, disease-free survival, and EEC-specific survival were p = 0.131, p = 0.068, and p = 0.987, respectively.
Conclusion
MSI is likely an important biomarker for poor prognosis in patients with EEC, and this correlation is even more certain in patients with early-stage EEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer (EC) is one of the most common cancers of the female reproductive tract. Its incidence and mortality are increasing annually, and an increasing number of patients present with cancer progression and recurrence [1, 2].
EC is classified into two types, type I and type II, based on histological and clinicopathological features. Type I EC, known as endometrioid endometrial cancer (EEC), accounts for the majority of EC cases (approximately 70–80%) [3]. Therefore, an increasing number of studies are focusing on the treatment and prognostic assessment of EEC, and the key to the prognostic assessment is the ability to identify a prognostic biomarker.
Microsatellite instability (MSI) is present in approximately 20–40% of patients with sporadic EEC [4, 5], so that many studies focused on correlation between MSI and prognosis in patients with EEC [6,7,8,9,10,11].
However, the studies on the correlation between MSI and EEC prognosis are currently divided, with some studies concluding that MSI has no significant correlation with the prognosis of EEC, some studies concluding that MSI is a biomarker for a good prognosis of EEC, and other studies concluding that MSI is a biomarker for a poor prognosis of EEC. However, a uniform conclusion has not been established and no relevant meta-analysis has been reported.
We conducted this systematic review and meta-analysis to clarify the correlation between MSI and the prognosis of EEC.
Methods
Data sources and search strategy
This meta-analysis was rigorously evaluated using the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidelines [12]. PubMed, EMBASE, and the Cochrane Collaboration Library databases were searched from inception to July 2021, and the language was restricted to English.
We adjusted the MeSH terms combined with related text words to comply with the relevant rules for searching for relevant studies in each database. Our search strategy was as follows: (Endometrial Neoplasm or Endometrial Neoplasms or Endometrial Carcinoma or Endometrial Carcinomas or Endometrial Cancer or Endometrial Cancers or Endometrium Cancer or Cancer of the Endometrium or Carcinoma of Endometrium or Endometrium Carcinoma or Endometrium Carcinomas or Cancer of Endometrium or Endometrium Cancers) AND (Mismatch repair or Microsatellite instability or Replication Error Phenotype or Replication Error Phenotypes) AND survival.
Study selection
Two independent researchers (Jing-ping Xiao and Yun-zi Wang) filtered all the titles and abstracts of the retrieved studies to identify potentially relevant studies. The full texts of the retrieved studies that met the inclusion criteria were evaluated. Each of these discrepancies was resolved through discussion, and if conflicts remained, a third reviewer (Ji-sheng Wang) was involved.
Inclusion and exclusion criteria
Studies describing the correlation between MSI and the prognosis of EEC were included if they met the following criteria: (1) patients with EEC or early-stage EEC (stage I-II); (2) reported overall survival, disease-free survival, progression-free survival, EEC-specific survival, or recurrence-free survival associated with MSI or mismatch repair deficiency; (3) directly reported HR or OR with 95% CI or generated Kaplan–Meier survival curves that could be used to extract HR.
Editorials, meeting reports, and letters to the editors were all excluded.
Data extraction
Two researchers (Jing-ping Xiao and Yun-zi Wang) independently screened studies based on the inclusion criteria, and any differences were resolved by consensus. From each study, we extracted the study characteristics, baseline characteristics, and pre-established outcomes for overall survival, disease-free survival, progression-free survival, EEC-specific survival, or recurrence-free survival.
Definition of MSI
MSI was defined as a lack of expression of at least 1 of the mismatch repair proteins (MLH1, MSH2, PMS2, and MSH6) detected using immunohistochemistry [11, 13, 14]; alternatively, microsatellite markers were identified by DNA isolation and molecular analysis, and tumors were considered to present MSI when they showed alterations in at least 2 of the 3–6 markers or in at least 1 of the 2 markers [7, 15,16,17,18,19].
Quality assessment
Two researchers (Jing-ping Xiao and Yun-zi Wang) separately applied the Newcastle–Ottawa Statement [20] to evaluate the quality of eligible studies, including selection, comparability, and exposure. Nine points were included in the scale, and a score greater than or equal to 7 was considered a high-quality study. A score of 4–6 was considered a good-quality study, a score of 3 or less was considered a low-quality study, and discrepancies were resolved through discussion, with the involvement of a third reviewer (Ji-sheng Wang) if a conflict remained.
Data synthesis and analysis
Stata (version 14) software was used to analyze all results. The HRs were extracted and calculated from Kaplan–Meier survival curves if HRs were not directly reported in the study. An I2-value greater than or equal to 50% indicated significant heterogeneity, and then the HR were merged with the corresponding 95% CI using a random-effects model; otherwise, the fixed-effects model was used. Publication bias was statistically assessed using Egger’s regression test and Begg’s test, where a p-value < 0.05 was considered to indicate significant publication bias.
Results
Literature search
Figure 1 illustrates the flow chart for the selection of eligible studies. 720 studies were identified by searching PubMed, Cochrane, and EMBASE databases. 469 studies remained after removing duplicate files. After scanning the titles and abstracts, 50 studies were selected for full-text review. Finally, we included 17 studies that met the inclusion criteria for our meta-analysis [7, 8, 11, 13,14,15,16,17,18,19, 21,22,23,24,25,26,27].
Study characteristics
Table 1 shows the characteristics of the 17 studies included. Of these studies, 7 studies were conducted in Europe (Spain, Italy, Norway, and the European region) [8, 11, 13, 15, 21, 22, 24], and 9 studies were conducted in the Americas (United States, Canada, and North American region) [7, 14, 16,17,18,19, 23, 24, 27]. 1 study was conducted in Asia (Korea) [25], and 1 study was conducted in Oceania (Australia) [26]. 15 studies were retrospective cohort studies [8, 11, 13,14,15,16,17,18,19, 21, 23,24,25,26,27], and 2 studies were clinical trials [7, 22]. 8 studies assessed MSI using quasimonomorphic mononucleotide markers [7, 8, 15,16,17,18,19, 21], and 9 studies assessed MSI using immunohistochemistry [11, 13, 14, 22,23,24,25,26,27].
As shown in Table 2, all studies scored 7 or higher and were high-quality studies.
Correlation between MSI and overall survival in the EEC or early-stage EEC
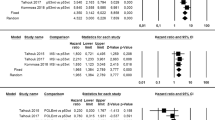
The pooled HR for patients with EEC showed that MSI was significantly associated with shorter overall survival [HR = 1.37, 95% CI (1.00–1.86), p = 0.048]. Meanwhile, significant heterogeneity was observed (I2 = 60.6%), as shown in Fig. 2a.
In the subgroup analysis of patients with early-stage EEC, patients with MSI had a shorter overall survival [HR = 1.47, 95% CI (1.11–1.95), p = 0.07], and no heterogeneity was observed (I2 = 20.9%), as shown in Fig. 3a.
Correlation between MSI and disease-free survival in the EEC or early-stage EEC
The pooled HR for patients with EEC showed that MSI was associated with shorter disease-free survival [HR = 1.99, 95% CI (1.31–3.01), p = 0.000]. Meanwhile, heterogeneity was observed (I2 = 65.7%, p = 0.001), as shown in Fig. 2b.
In the subgroup analysis of early-stage EEC, patients with MSI had a shorter disease-free survival [HR = 4.17, 95% CI (2.37–7.41), p = 0.000], and no heterogeneity was identified (I2 = 0.0%), as shown in Fig. 3b.
Correlation between MSI and EEC-specific survival in patients with EEC
As shown in Fig. 2c, the pooled HR for patients with EEC showed a significant association between MSI with shorter EEC-specific survival [HR = 2.07, 95% CI (1.35–3.18), p = 0.001]. Meanwhile, no significant heterogeneity was observed (I2 = 31.6%).
Correlation between MSI and progression-free survival in patients with early-stage EEC
As shown in Fig. 3c, the pooled HR for the early-stage EEC subgroup showed that MSI was significantly associated with shorter progression-free survival [HR = 2.41, 95% CI (1.05–5.54), p = 0.039]. Meanwhile, no heterogeneity was detected (I2 = 0.0%).
Correlation between MSI and recurrence-free survival in patients with EEC
The pooled HR for patients with EEC showed that MSI was not significantly associated with shorter recurrence-free survival [HR = 1.35, 95% CI (0.27–6.60), p = 0.714]. Meanwhile, significant heterogeneity was observed (I2 = 92.7%) (Supplemental Fig. 1).
Correlation between MSI and recurrence rate in patients with EEC
The pooled OR for EEC showed that MSI was significantly associated with a higher recurrence rate [OR = 2.72, 95% CI (1.56–4.76), p = 0.000]. Heterogeneity in the recurrence rate was not observed (I2 = 0.0%) (Supplemental Fig. 2).
Publication bias
No significant publication bias was detected in the funnel plot (Supplemental Fig. 3). Additionally, significant publication bias was not observed, and the p-values of Egger’s test for overall survival, disease-free survival, and EEC-specific survival were significant (p = 0.131, p = 0.068, and p = 0.987, respectively).
Sensitivity analysis
We omitted each study individually from the pooled analysis to explore the sensitivity of the pooled HR for overall survival, disease-free survival, and progression-free survival in EEC. The exclusion of any study did not have a significant effect on the results (Supplemental Fig. 4).
Discussion
Classical parameters associated with a high risk of EC recurrence include FIGO stage, age, histological tumor type and grade, depth of myometrial infiltration, and presence of lymphovascular infiltration, however they do not accurately predict the prognosis of EC [28].
Therefore, The Cancer Genome Atlas (TCGA) classified EC into 4 types [29], POLE mutant, MSI, low-copy, and high-copy types, according to their molecular characteristics to better identify patients at high risk of recurrence and to allow appropriate treatment or follow-up of patients and to avoid overtreatment of patients with good prognosis.
MSI, as one of the TCGA strains of endometrial cancer, is mostly found in EEC, and its correlation with EEC prognosis has been a recent research hotspot. It has been hypothesized that MSI can lead to altered immune surveillance in endometrial cancer as well as lead to altered host cell-cancer cell interactions, which may determine the prognosis of EEC patients with MSI [30], and it has also been found that EEC patients with MSI have higher tumor grade and are more prone to retroperitoneal lymph node recurrence [31]. Although some studies [9] showed that there was no significant correlation between MSI and the prognosis of EEC patients. However, our combined analysis of studies related to MSI and EEC patients found that there was indeed a strong association between MSI and EEC.
In our meta-analysis, Patients with MSI had a significantly poorer prognosis in terms of overall survival, disease-free survival, EEC-specific survival, and the recurrence rate. However, there was significant heterogeneity in the pooled data for overall survival and disease-free survival. And we performed sensitivity analyses and did not detect studies that caused heterogeneity.
Due to patients with early-stage EEC rarely receive adjuvant therapy, we performed a subgroup analysis of the prognostic value of MSI in patients with early-stage EEC. The pooled analysis showed that Patients with MSI had a significantly poor prognosis in terms of overall survival, disease-free survival, and progression-free survival, and the heterogeneity disappeared in all pooled data.
In addition, in our meta-analysis, there was no significant correlation was observed between MSI and recurrence-free survival. The pooled analysis showed that MSI was associated with better recurrence-free survival in the study by Bosse et al. [24]. We found that EEC was FIGO grade 3 only in this study, the most patients received adjuvant therapy. In contrast, in the study by Backes et al. [27], MSI was associated with a significantly worse recurrence-free survival, in which EEC was FIGO grade1-3, and patients receive adjuvant at a much lower rate. Therefore, We assume that more adjuvant therapy is an important influencing factor on the prognostic value of MSI.
To more accurately predict the prognosis of EC patients, currently, the ESTRO/ESGO/ESP guidelines recommend a risk stratification system using a combination of TCGA molecular typing and classical clinicopathological factors for the management of EC patient [32]. And the accuracy of prediction would be enhanced if the TCGA molecular typing and classical clinicopathological factors were independent from each other.
There were some studies had demonstrated that, in EC patients, some classical clinicopathological factors, such as LVSI, have prognostic value independent of TCGA markers, age, and adjuvant treatment [33, 34]. And the effect of deep myometrial invasion (DMI) on the risk of recurrence is independent from the TCGA group [35]. The additional study suggested that, in EC patients, some TCGA molecular typings, such as MSI, may predict independently lower disease-specific survival [36]. And our findings also suggest that MSI may be an independent prognostic factor for EEC. Of course, as previously discussed, more studies to confirm whether other TCGA molecular typing and classical clinicopathological factors are independent of each other are necessary.
Our study has some limitations. First, some data were extracted from survival curves, which may produce some bias compared with the real data. Second, the number of studies on recurrence-free survival was small, and more studies are needed to support our conclusions. Third, the vast majority of studies we included were retrospective case studies, which carries the risk of selective reporting. However, the heterogeneity of the combined data for our meta-analysis was not significant generally, and no significant publication bias was detected in the included studies, so our general results were reliable.
Conclusion
In summary, MSI has a significant prognostic value in EEC, and this prognostic value is more definite in patients with early-stage EEC.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30
Rutgers JK (2015) Update on pathology, staging and molecular pathology of endometrial (uterine corpus) adenocarcinoma. Future Oncol 11(23):3207–3218
Soreide K, Janssen EA, Soiland H et al (2006) Microsatellite instability in colorectal cancer. Br J Surg 93(4):395–406
Akagi K, Oki E, Taniguchi H et al (2021) The real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci 112(3):1105–1113
Black D, Soslow RA, Levine DA et al (2006) Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol 24(11):1745–1753
Mackay HJ, Gallinger S, Tsao MS et al (2010) Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur J Cancer 46(8):1365–1373
Steinbakk A, Malpica A, Slewa A et al (2011) Biomarkers and microsatellite instability analysis of curettings can predict the behavior of FIGO stage I endometrial endometrioid adenocarcinoma. Mod Pathol 24(9):1262–1271
Diaz-Padilla I, Romero N, Amir E et al (2013) Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 88(1):154–167
Kim SR, Pina A, Albert A et al (2018) Does MMR status in endometrial cancer influence response to adjuvant therapy? Gynecol Oncol 151(1):76–81
Ruz-Caracuel I, Ramon-Patino JL, Lopez-Janeiro A et al (2019) Myoinvasive pattern as a prognostic marker in low-grade, early-stage endometrioid endometrial carcinoma. Cancers 11(12):1845
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Nout RA, Bosse T, Creutzberg CL et al (2012) Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K-AKT, Wnt/beta-catenin and P53 pathway activation. Gynecol Oncol 126(3):466–473
Kim SR, Pina A, Albert A et al (2020) Mismatch repair deficiency and prognostic significance in patients with low-risk endometrioid endometrial cancers. Int J Gynecol Cancer 30(6):783–788
Fiumicino S, Ercoli A, Ferrandina G et al (2001) Microsatellite instability is an independent indicator of recurrence in sporadic stage I–II endometrial adenocarcinoma. J Clin Oncol 19(4):1008–1014
Maxwell GL, Risinger JI, Alvarez AA et al (2001) Favorable survival associated with microsatellite instability in endometrioid endometrial cancers. Obstet Gynecol 97(3):417–422
Zighelboim I, Goodfellow PJ, Gao F et al (2007) Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol 25(15):2042–2048
Bilbao C, Lara PC, Ramirez R et al (2010) Microsatellite instability predicts clinical outcome in radiation-treated endometrioid endometrial cancer. Int J Radiat Oncol Biol Phys 76(1):9–13
Zighelboim I, Ali S, Lankes HA et al (2015) Assessing the prognostic role of ATR mutation in endometrioid endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 138(3):614–619
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Bilbao-Sieyro C, Ramirez R, Rodriguez-Gonzalez G et al (2014) Microsatellite instability and ploidy status define three categories with distinctive prognostic impact in endometrioid endometrial cancer. Oncotarget 5(15):6206–6217
Ruiz I, Martin-Arruti M, Lopez-Lopez E et al (2014) Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol Oncol 134(1):20–23
McMeekin DS, Tritchler DL, Cohn DE et al (2016) Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol 34(25):3062–3068
Bosse T, Nout RA, McAlpine JN et al (2018) Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol 42(5):561–568
Kim J, Kong JK, Yang W et al (2018) DNA mismatch repair protein immunohistochemistry and MLH1 promotor methylation testing for practical molecular classification and the prediction of prognosis in endometrial cancer. Cancers 10(9):279
Nagle CM, O’Mara TA, Tan Y et al (2018) Endometrial cancer risk and survival by tumor MMR status. J Gynecol Oncol 29(3):e39
Backes FJ, Haag J, Cosgrove CM et al (2019) Mismatch repair deficiency identifies patients with high-intermediate-risk (HIR) endometrioid endometrial cancer at the highest risk of recurrence: a prognostic biomarker. Cancer 125(3):398–405
Bendifallah S, Canlorbe G, Collinet P et al (2015) Just how accurate are the major risk stratification systems for early-stage endometrial cancer? Br J Cancer 112(5):793–801
Travaglino A, Raffone A, Mollo A, Borrelli G, Alfano P, Zannoni GF et al (2020) TCGA molecular subgroups and FIGO grade in endometrial endometrioid carcinoma. Arch Gynecol Obstet 301(5):1117–1125
McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA et al (2016) Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol 34(25):3062–3068
Kim SR, Tone A, Kim RH, Cesari M, Clarke BA, Eiriksson L et al (2021) Understanding the clinical implication of mismatch repair deficiency in endometrioid endometrial cancer through a prospective study. Gynecol Oncol 161(1):221–227
Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S et al (2021) ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31(1):12–39
Raffone A, Travaglino A, Raimondo D, Neola D, Maletta M, Santoro A et al (2022) Lymphovascular space invasion in endometrial carcinoma: a prognostic factor independent from molecular signature. Gynecol Oncol 165(1):192–197
Travaglino A, Raffone A, Stradella C, Esposito R, Moretta P, Gallo C et al (2020) Impact of endometrial carcinoma histotype on the prognostic value of the TCGA molecular subgroups. Arch Gynecol Obstet 301(6):1355–1363
Raffone A, Travaglino A, Raimondo D, Neola D, Renzulli F, Santoro A et al (2021) Prognostic value of myometrial invasion and TCGA groups of endometrial carcinoma. Gynecol Oncol 162(2):401–406
Pasanen A, Loukovaara M, Butzow R (2020) Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod Pathol 33(7):1443–1452
Acknowledgements
With thanks to all participants in this study.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JPX and YZW designed the study and wrote the manuscript. YYZ and JD developed the search strategy and completed the literature search. JSW developed the inclusion and exclusion criteria for the eligible studies. JPX, YZW, and JSW reviewed the eligible studies and extracted the data. JPX and YYZ did the methodological judgement. YYZ and JD performed the statistical analysis methods. JPX, YZW, and JD summarized the original data. Contributions to the interpretation of the data and review of the manuscript were made by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, Jp., Wang, Js., Zhao, Yy. et al. Microsatellite instability as a marker of prognosis: a systematic review and meta-analysis of endometrioid endometrial cancer survival data. Arch Gynecol Obstet 307, 573–582 (2023). https://doi.org/10.1007/s00404-022-06636-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06636-8