Abstract
Background
The Cancer Genome Atlas (TCGA) identified four prognostic subgroups of endometrial carcinoma: copy-number-low/p53-wild-type (p53wt), POLE-mutated/ultramutated (POLEmt), microsatellite-instability/hypermutated (MSI), and copy-number-high/p53-mutated (p53mt). However, it is still unclear if they may be integrated with the current histopathological prognostic factors, such as histotype.
Objective
To assess the impact of histotype on the prognostic value of the TCGA molecular subgroups of endometrial carcinoma.
Methods
A systematic review and meta-analysis was performed by searching 7 electronic databases from their inception to April 2019 for studies assessing prognosis in all TCGA subgroups of endometrial carcinoma. Pooled hazard ratio (HR) for overall survival (OS) was calculated in two different groups (“all-histotypes” and “endometrioid”), using p53wt subgroup as reference standard; HR for non-endometrioid histotypes was calculated indirectly. Disease-specific survival and progression-free survival were assessed as additional analyses.
Results
Six studies with 2818 patients were included. In the p53mt subgroup, pooled HRs for OS were 4.322 (all-histotypes), 2.505 (endometrioid), and 4.937 (non-endometrioid). In the MSI subgroup, pooled HRs were 1.965 (all-histotypes), 1.287 (endometrioid), and 6.361 (non-endometrioid). In the POLEmt subgroup, pooled HRs were 0.763 (all-histotypes), 0.481 (endometrioid), and 2.634 (non-endometrioid). Results of additional analyses were consistent for all subgroups except for non-endometrioid POLEmt carcinomas.
Conclusion
Histotype of endometrial carcinoma shows a crucial prognostic value independently of the TCGA molecular subgroup, with non-endometrioid carcinomas having a worse prognosis in each TCGA subgroup. Histotype should be integrated with molecular characterization for the risk stratification of patients in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial carcinoma is the most common gynecologic cancer in the Western world [1, 2]. In the last decades, both incidence and mortality of endometrial carcinoma have shown an increase [1]. Causes of such an unfavorable trend probably lie in an inaccurate risk stratification, which would cause many patients to be undertreated or overtreated [3, 4].
In 2013, The Cancer Genome Atlas (TCGA) Research Network has identified four novel molecular prognostic subgroups of endometrial carcinoma: copy-number-low/p53-wild-type (p53wt), POLE-mutated/ultramutated (POLEmt), microsatellite-instability/hypermutated (MSI), and copy-number-high/p53-mutated (p53mt) [5,6,7,8,9,10,11]. This reclassification has had great impact on the scientific research, since the TCGA subgroups have the potential of improving the risk stratification in endometrial carcinoma, with consequent improvement in the patient management [6,7,8,9,10,11]. Given the costs, complex equipment, and expertise required for sequencing techniques, the TCGA classification appears little applicable in the common practice; therefore, great efforts have been made in the search for cheaper surrogates of molecular markers [6,7,8,9,10,11,12,13]. The Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) has proposed the use of immunohistochemistry for mismatch repair proteins and p53 as surrogates of MSI assessment and TP53 sequencing, respectively [6, 8, 11]. Indeed, immunohistochemistry is cheaper, faster, and more widely available than sequencing techniques [14,15,16,17,18,19,20,21,22].
While the prognostic value of the TCGA subgroups has been confirmed in several studies [6,7,8,9,10,11], it is still unclear how they may be integrated with histologic features such as tumor grade and histotype. In fact, while some authors hypothesized that molecular features may completely replace histologic features in the future, other ones consider the value of histology as crucial for the patient management [23, 24].
The objective of this study was to assess if and how histotype affects the prognostic value of the TCGA subgroups of endometrial carcinoma. For this purpose, we calculated pooled hazard ratios (HR) for overall survival (OS), disease-specific survival (DSS), and progression-free survival (PFS) in each TCGA subgroup, assessing how they change based on histotype.
Materials and methods
Study protocol
Study methods were defined a priori. All stages were completed by two reviewers (AT, AR). Disagreements were resolved by discussion among all authors. The study was reported according to the Preferred Reporting Item for Systematic Reviews and Meta-analyses (PRISMA) statement [25].
Search strategy
Web of Sciences, Google Scholar, Scopus, MEDLINE, ClinicalTrial.gov, Cochrane Library, and EMBASE were used as electronic databases from their inception to April 2019. Several different combinations of the following text words were used: “survival”; “TP53″; “tumor protein 53”; “p53”; “endometr*”; “copy number”; “POLE”; “MMR”; “mismatch repair”; “MSI”; “EPCAM”; “microsatellite instability”; “MLH1”; “MSH2”; “MSH6”; “PMS2”; “ultramutated”; “hypermutated”; “cancer”; “carcinoma”; “adenocarcinoma”; “neoplas*”; “tumor”; “tumour”; “endometrioid”; “serous”; “clear cell”; “undifferentiated”; “immunohistochemistry”; “immunohistochemical”; “marker”; “prognosis”; “Atlas”; “cancer”; “genome”; “PORTEC”; “TransPORTEC”; “TCGA”; “ProMisE”; “Proactive Molecular Risk Classifier”. References from each study were also assessed.
Study selection
All peer-reviewed studies assessing prognosis in each TCGA subgroup of endometrial carcinoma were included. Exclusion criteria, defined a priori, were: sample size < 10 in any TCGA subgroup; minimal follow-up time < 2 years; case reports; reviews; overlapping patient data (in this case, the study with smaller sample size was excluded). Studies not assessing prognosis in any TCGA subgroup were also excluded.
Data extraction
Primary data extracted were HR estimates with 95% confidence interval (CI) for each TCGA subgroup.
To assess the impact of histotype on the prognosis of the TCGA subgroup, pooled HR was calculated separately for two group of studies: the first group was composed of studies that assessed endometrial carcinomas of any histotype (“all-histotypes” group), while the second group was composed of studies that assessed only endometrioid carcinomas (“endometrioid” group). HR from multivariate analyses were not considered, since they were normalized for all clinicopathological factors, not allowing to isolate the impact of histotype.
PICOS were used for data extraction as follows:
“P” (population) of our study was patients with endometrial carcinoma.
“I” (intervention or risk factor) was the TCGA subgroup (p53mt, MSI, POLEmt), assessed by molecular sequencing or immunohistochemical surrogates according to the ProMisE [2, 6, 19].
“C” (comparator) was the p53wt subgroup.
“O” (outcomes) were OS (primary outcome), and DSF and PFS (secondary outcomes). OS (or time to death) was defined as time from surgery until death of any cause. DSF (or time to death from disease) was defined as time from surgery until death due to endometrial cancer. PFS (or time to progression) was defined as time from surgery until there is evidence of recurrent or progressive disease (this is based on either clinical evidence of recurrence or imaging confirmation of recurrence) or if they died of the disease prior to the censoring date.
“S” (study design) was cohort study.
Assessment of risk of bias within studies
The Methodological Index for Non-Randomized Studies (MINORS) was used to assess the risk of bias within studies [26]. Six domains related to risk of bias were assessed in each study: (1) Aim (i.e., clearly stated aim); (2) Patients (i.e., all eligible patients were included in the study during the period of enrollment); (3) Data (i.e., data were collected according to a protocol defined before the beginning of the study); (4) Endpoint (i.e., clear explanation of methods used for outcomes measurement); (5) Bias (i.e., the study endpoints were blindly evaluated, re-evaluated, or evaluated by two or more authors); (6) Follow-up (i.e., follow-up time of at least 2 years).
Authors’ judgments were categorized as “low risk”, “unclear risk”, or “high risk” of bias as previously described [27,28,29,30,31].
Data analysis
Hazard ratio (HR) with 95% CI for each TCGA subgroup was extracted from each study and pooled by using the random effect model of DerSimonian and Laird. HR values with 95% CI were reported for each study and as pooled estimated on forest plots.
HR analysis was performed separately in “all-histotypes” group and “endometrioid” group. Pooled HR of non-endometrioid histotypes was calculated indirectly using the following equation for each TCGA subgroup:
Here, n is the number of carcinomas; HR all histotype, n all histotypes, n endometrioid, and n non-endometrioid were extracted from the “all-histotypes” group of studies.
The number of endometrial carcinomas of the “endometrioid” group was excluded from the equation, because it would have altered the proportion among the prevalence of the different histotypes in the four TCGA groups.
Statistical heterogeneity among studies was quantified through the inconsistency index I2: heterogeneity was categorized as: null for I2 = 0%, minimal for I2 < 25%, low for I2 < 50%, moderate for I2 < 75%, and high for I2 ≥ 75%, as previously described [32,33,34,35,36,37,38,39].
Data analysis was performed using Comprehensive Meta-Analysis (Biostat,14 North Dean Street, Englewood, NJ 07631, USA) and Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration, 2014).
Results
Study selection
Six studies with a total of 2818 patients diagnosed with endometrial carcinomas were included [6,7,8,9,10,11]. The whole process of study selection is shown in Supplementary Fig. 1.
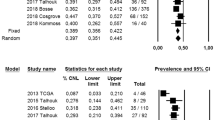
The patient cohort was retrospective in three studies [6,7,8], prospective in one study [10], derived from a randomized-controlled trial (RCT) in one study [7], and mixed (RCT + retrospective) in the remaining study [9]. Three studies were included in the “all-histotypes” group [6, 8, 11] and three in the “endometrioid” group [7, 9, 10]. In the “all-histotypes” group, the prevalence of non-endometrioid histotypes was 4% in the p53wt subgroup, 74.7% in the p53mt subgroup, 13.4% in the MSI subgroup, and 13.1% in the POLEmt subgroup.
Characteristics of the included studies are shown in Table 1.
Risk of bias within studies
For the “aim”, “data”, “endpoint”, and “follow-up” domains, all studies were considered at low risk of bias.
For the “patients” domain, two studies were considered at unclear risk of bias, because they did not clearly state if patients were consecutively selected [6, 8]. All the remaining studies were considered at low risk of bias.
For the “bias” domain, one study was considered at unclear risk of bias, since it was unclear if specimens were blindly evaluated, re-evaluated, or evaluated by two or more authors; all the other studies were considered at low risk of bias [9].
Results about risk of bias assessment are shown in Supplementary Fig. 2.
Main analysis
All included studies assessed OS and thus were suitable for the main analysis [6,7,8,9,10,11].
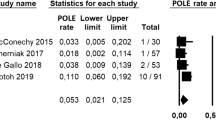
In the “all-histotypes” group, pooled HR was 4.322 (95% CI 3-6.226; I2 = 16,89) for p53mt subgroup, 1.965 (95% CI 1.384–2.789; I2 = 0) for MSI subgroup, and 0.763 (95% CI 0.426–1.367; I2 = 0) for POLEmt subgroup (Fig. 1).
In the “endometrioid” group, pooled HR was 2.505 (95% CI 1.119–5.609; I2 = 90,69) for p53mt subgroup, 1.287 (95% CI 0.793–2.089; I2 = 81,36) for MSI subgroup, and 0.481 (95% CI 0.231–1.004; I2 = 37,29) for POLEmt subgroup (Fig. 2).
The HR estimate for non-endometrioid histotypes was 4.937 for p53mt subgroup, 6.361 for MSI subgroup, and 2.634 for POLEmt subgroup.
Additional analyses
In the “all-histotypes” group, all studies assessed DSS and PFS and, thus, were suitable for additional analyses. With regard to DSS, pooled HR was 5.493 (95% CI 3.045–9.908; I2 = 49,1) for p53mt group, 2.244 (95% CI 1.211–4.157; I2 = 25,38) for MSI group, and 0.565 (95% CI 0.247–1.291; I2 = 0) for POLEmt group (Supplementary Fig. 3). With regard to PFS, pooled HR was 5.509 (95% CI 2.513–12.078; I2 = 0) for p53mt group, 1.85 (95% CI 0.905–3.78; I2 = 84,24) for MSI group, and 0.397 (95% CI 0.162–0.971; I2 = 0) for POLEmt group (Supplementary Fig. 4).
In the “endometrioid” group, only 1 study assessed DSS [10], and only 2 assessed PFS [9, 10]. With regard to DSS, pooled HR was 3.95 (95% CI 2.099–7.435; I2 = 0) for p53mt group, 1.58 (95% CI 0.919–2.717; I2 = 0) for MSI group, and 0.48 (95% CI 0.063–3.671; I2 = 0) for POLEmt group (Supplementary Fig. 5). With regard to PFS, pooled HR was 2.031 (95% CI 1.494–2.762; I2 = 0) for p53mt group, 0.978 (95% CI 0.476–2.012; I2 = 84,24) for MSI group, and 0.207 (95% CI 0.084–0.51; I2 = 0) for POLEmt group (Supplementary Fig. 6).
In non-endometrioid histotypes, the HR estimate for DSS was 6.016 for p53mt subgroup, 6.549 for MSI subgroup, and 1.129 for POLEmt subgroup. The estimated HR for PFS was 6.687 for p53mt subgroup, 7.504 for MSI subgroup, and 1.658 for POLEmt subgroup.
Scales of HR values for OS, DSS, and PFS are summarized in Fig. 3, Supplementary Fig. 7, and Supplementary Fig. 8, respectively.
Discussion
Main analysis
Our study showed that, although the prognostic value of TCGA subgroups was confirmed, prognosis was affected by histotype in each TCGA prognostic subgroup.
In accordance with the initial TCGA findings, the overall prognosis of the p53mt subgroup was by far the worst among the four TCGA subgroups [5, 40]. Considering all histotypes, the HR for OS was indeed 4.3, indicating a prognosis more than four times worse than that of the p53wt subgroup. Considering only endometrioid histotypes, the HR showed an important decrease, with a value of 2.5. On the other hand, the HR estimate for non-endometrioid histotypes went up to about 5. This indicates a strong impact of non-endometrioid histotypes on the overall prognosis of the p53mt group. Such impact appeared clearly stronger than that in the other subgroups. In fact, as above mentioned, the p53mt subgroup is mainly composed of serous carcinomas [5,6,7,8,9,10,11]. For this reason, the p53mt subgroup was also referred to as “serous” subgroup by the TCGA [5]. These findings suggest that non-endometrioid histotypes account for an important part of the worse prognosis of the p53mt subgroup. However, although decreased, the unfavorable prognostic value of TP53 mutations remained significant even considering only endometrioid histotypes. In this regard, it may be appropriate to remark that an aberrant p53 expression does not imply a diagnosis of serous carcinoma, given the existence of non-serous p53mt carcinomas and the difference in the prognosis of p53mt carcinomas based on the histotype [41,42,43,44].
The MSI subgroup showed the second worst overall prognosis after the p53mt subgroup, although with HR values definitely lower than those in the latter one. This appears consistent with the percentage of non-endometrioid histotypes, which is much higher than that in the p53wt subgroup, but much lower than that in the p53mt subgroup. Considering all histotypes, the HR value was about 2, indicating that the prognosis of the MSI subgroup is about two times worse than that of the p53wt subgroup. Considering only endometrioid histotype, the HR value decreased to 1.3, indicating a prognosis slightly worse than that of the p53wt subgroup, which did not reach a statistically significant difference. For non-endometrioid histotypes, the HR increased to over 6, indicating a prognosis even worse than that of non-endometrioid p53mt carcinomas. This outstanding result might be explained by the high frequency of MSI in highly aggressive histotypes such as undifferentiated and dedifferentiated carcinomas [45,46,47,48]. This once again supported the importance of histotype in the risk stratification. Therefore, the suggestion that mismatch repair deficiency and microsatellite instability imply a diagnosis of endometrioid histotype appears inappropriate [24]. However, the similar prognosis between endometrioid MSI carcinomas and p53wt carcinomas does not imply that the MSI subgroup has not its own prognostic value. Indeed, despite being homogeneous about histotype, the p53wt subgroup is genetically heterogeneous and lacks a molecular or immunohistochemical signature [5,6,7,8,9,10,11]. There is evidence that the p53wt subgroup can be subdivided in at least two sub-subgroups, based on the presence of CTNNB1 exon 3 mutations [12, 23]. In this regard, it has been shown that the sub-subgroup with CTNNB1 may have a prognosis similar to that of the MSI subgroup [7, 12, 23]. This would explain the prognostic overlap observed in our analysis.
In the POLE subgroup, the overall prognosis was the best one, with an HR of about 0.7, indicating a prognosis slightly better than that of the p53wt subgroup. However, such difference was not statistically significant. Considering only endometrioid histotype, the difference with the p53wt group even increased, with a prognosis more than two times better than that of the p53wt subgroup. On the other hand, in non-endometrioid carcinomas, the prognosis of the POLE subgroup sensibly worsened, with an HR of 2.6; this indicates a prognosis of more than two times and half worse than that of the p53wt subgroup, and even worse than that of endometrioid p53mt carcinomas. Therefore, even in the POLEmt subgroup, histotype seems to have a crucial prognostic value. This appears in contrast with the assumptions that all POLEmt carcinomas should be diagnosed as endometrioid, or that they are not affected by histotype [24].
The scale of HR values reported in Fig. 3 suggests that non-endometrioid carcinomas have a risk of death of any cause worse than that of endometrioid carcinomas, regardless of the TCGA subgroup. However, at the same histotype, the prognosis was very different amongst TCGA subgroup. These findings endorse the efforts for an integration of molecular data with clinicopathological factors in the risk stratification of endometrial cancer [49,50,51,52,53,54,55], to achieve an optimal and tailored patient management.
Additional analyses
Analyses of DSS and PFS partially confirmed the findings of the OS analysis, with non-endometrioid MSI carcinomas showing the worst prognosis, followed by non-endometrioid p53mt carcinomas, and with endometrioid POLEmt carcinomas showing the best prognosis.
The main difference lied in the prognosis of non-endometrioid POLEmt carcinomas. Indeed, while the risk of death of any cause was similar to that of endometrioid p53mt carcinomas, the risk of death by cancer was definitely lower, being similar to that of the p53wt subgroup. It might be hypothesized that non-endometrioid POLEmt carcinomas occur preferentially in patients older and/or with more comorbidities if compared to endometrioid POLEmt carcinomas; further studies are necessary to investigate this point. On the other hand, in this subset, the risk of recurrence/progression was intermediate between p53wt subgroup and endometrioid p53mt.
Endometrioid p53mt carcinomas still showed a risk definitely lower than their non-endometrioid counterpart (two times lower for DSS and more than three times lower for PFS).
Regarding endometrioid MSI carcinomas, they still showed a risk higher than p53wt subgroup in DSS analysis, although without statistical significance, while the risk of recurrence/progression was similar.
Strengths and limitations
To the best of our knowledge, this may be the first meta-analysis assessing the prognostic value of histotype regarding the four TCGA molecular subgroups of endometrial cancer. This study provided estimates on the prognosis of each TCGA subgroup separately for endometrioid and non-endometrioid carcinomas. Our results are also strengthened by the reliability of the reference standard about histotype. Indeed, given the low prevalence of non-endometrioid histotypes in the p53wt subgroup, its prognosis was not expected to significantly change between the “all-histotypes” and the “endometrioid” group.
Limitations of our meta-analysis might be the indirect calculation of HR for non-endometrioid histotypes and the impossibility of calculating HR in each non-endometrioid histotype (serous, mucinous, clear cell, and undifferentiated/dedifferentiated, carcinosarcoma).
Conclusion
In endometrial carcinoma, histotype maintains a crucial prognostic value independently from the TCGA molecular subgroups, with non-endometrioid carcinomas having a worse prognosis in each subgroup.
In particular, among all TCGA subgroups, non-endometrioid carcinomas of the MSI subgroup have the highest risk of death of any cause, death by cancer, and recurrence/progression of disease, followed by non-endometrioid carcinomas of the p53mt subgroup; non-endometrioid POLEmt carcinomas showed a variable prognosis instead.
On the other hand, endometrioid carcinomas of the p53mt subgroup showed the worst prognosis among all endometrioid carcinomas, while endometrioid POLEmt carcinomas consistently showed the best prognosis among all endometrial carcinomas; endometrioid MSI carcinomas showed a significant overlap with the p53wt group.
Given these findings, histotype remains as a major prognostic factor in endometrial carcinoma, and it should be integrated with molecular characterization for the risk stratification. Further studies are necessary in this regard.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Travaglino A, Raffone A, Mascolo M et al (2019) TCGA Molecular subgroups in endometrial undifferentiated/dedifferentiated carcinoma. Pathol Oncol Res. https://doi.org/10.1007/s12253-019-00784-0(Epub ahead of print)
Gilks CB, Oliva E, Soslow RA (2013) Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 37:874–881
Hoang LN, McConechy MK, Kobel M et al (2013) Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 37:1421–1432
Cancer Genome Atlas Research Network et al (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73
Talhouk A, McConechy MK, Leung S et al (2015) A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 113(2):299–310
Stelloo E, Nout RA, Osse EM et al (2016) Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 22(16):4215–4224
Talhouk A, McConechy MK, Leung S et al (2017) Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 123(5):802–813
Bosse T, Nout RA, McAlpine JN et al (2018) Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol 42(5):561–568
Cosgrove CM, Tritchler DL, Cohn DE et al (2018) An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol Oncol 148(1):174–180
Kommoss S, McConechy MK, Kommoss F et al (2018) Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 29(5):1180–1188
Travaglino A, Raffone A, Saccone G et al (2019) Immunohistochemical nuclear expression of β-catenin as a surrogate of CTNNB1 exon 3 mutation in endometrial cancer. Am J Clin Pathol 151(5):529–538
Kim G, Kurnit KC, Djordjevic B et al (2018) Nuclear β-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Mod Pathol 31:1553–1559
Raffone A, Travaglino A, Saccone G et al (2019) Should progesterone and estrogens receptors be assessed for predicting the response to conservative treatment of endometrial hyperplasia and cancer? A systematic review and meta-analysis. Acta Obstet Gynecol Scand. https://doi.org/10.1111/aogs.13586(Epub ahead of print)
Raffone A, Travaglino A, Mascolo M, Insabato L, Zullo F (2020) Predictive accuracy of hormone receptors in conservatively treated endometrial hyperplasia and early endometrioid carcinoma. Acta Obstet Gynecol Scand 99(1):140
Travaglino A, Raffone A, Saccone G et al (2018) PTEN as a predictive marker of response to conservative treatment in endometrial hyperplasia and early endometrial cancer. A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 231:104–110
Travaglino A, Raffone A, Saccone G et al (2019) Immunohistochemical predictive markers of response to conservative treatment of endometrial hyperplasia and early endometrial cancer: a systematic review. Acta Obstet Gynecol Scand. https://doi.org/10.1111/aogs.13587(Epub ahead of print)
Raffone A, Travaglino A, Saccone G et al (2019) PTEN expression in endometrial hyperplasia and risk of cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 299(6):1511–1524
Raffone A, Travaglino A, Saccone G et al (2019) PAX2 in endometrial carcinogenesis and in differential diagnosis of endometrial hyperplasia. A systematic review and meta-analysis of diagnostic accuracy. Acta Obstet Gynecol Scand 98(3):287–299
Travaglino A, Raffone A, Saccone G et al (2019) PTEN immunohistochemistry in endometrial hyperplasia: which are the optimal criteria for the diagnosis of precancer? APMIS 127(4):161–169
Raffone A, Travaglino A, Saccone G et al (2019) Loss of PTEN expression as diagnostic marker of endometrial precancer: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 98(3):275–286
Raffone A, Travaglino A, D’Antonio A et al (2020) BAG3 expression correlates with the grade of dysplasia in squamous intraepithelial lesions of the uterine cervix. Acta Obstet Gynecol Scand 99(1):99–104
McAlpine J, Leon-Castillo A, Bosse T (2018) The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol 244(5):538–549
Köbel M, Nelson GS (2018) Letter in response to: McAlpine J, Leon-Castillo A, Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol 2018; 244: 538–549. J Pathol 245:249–250
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (minors): development and validation of a newinstrument. ANZ J Surg 73(9):712–716
Raffone A, Travaglino A, Saccone G et al (2019) Endometrial hyperplasia and progression to cancer: which classification system stratifies the risk better? A systematic review and meta-analysis. Arch Gynecol Obstet 299(5):1233–1242
Raffone A, Travaglino A, Saccone G et al (2019) Management of women with atypical polypoid adenomyoma of the uterus: a quantitative systematic review. Acta Obstet Gynecol Scand. https://doi.org/10.1111/aogs.13553(Epub ahead of print)
Raffone A, Travaglino A, Saccone G et al (2019) Diabetes mellitus is associated with occult cancer in endometrial hyperplasia. Pathol Oncol Res. https://doi.org/10.1007/s12253-019-00684-3(Epub ahead of print)
Travaglino A, Raffone A, Saccone G et al (2019) Complexity of glandular architecture should be reconsidered in the classification and management of endometrial hyperplasia. APMIS 127(6):427–434
Travaglino A, Raffone A, Saccone G et al (2019) Congruence between 1994 who classification of endometrial hyperplasia and endometrial intraepithelial neoplasia system. Am J Clin Pathol. https://doi.org/10.1093/ajcp/aqz132(Epub ahead of print)
Travaglino A, Raffone A, Saccone G et al (2019) Endometrial hyperplasia and risk of coexistent cancer: WHO vs EIN criteria. Histopathology 74(5):676–687
Raffone A, Travaglino A, Saccone G et al (2019) Diabetes mellitus and responsiveness of endometrial hyperplasia and early endometrial cancer to conservative treatment. Gynecol Endocrinol. https://doi.org/10.1080/09513590.2019.1624716(Epub ahead of print)
Lionetti R, De Luca M, Travaglino A et al (2019) Treatments and overall survival in patients with Krukenberg tumor. Arch Gynecol Obstet 300(1):15–23
Raffone A, Travaglino A, Saccone G et al (2019) Diagnostic and prognostic value of ARID1A in endometrial hyperplasia: a novel marker of occult cancer. APMIS 127:597–606
Travaglino A, Raffone A, Mascolo M et al (2019) Clear cell endometrial carcinoma and the TCGA classification. Histopathology. https://doi.org/10.1111/his.13976(Epub ahead of print)
Raffone A, Travaglino A, Santoro A et al (2019) Accuracy of one-step nucleic acid amplification in detecting lymph node metastases in endometrial cancer. Pathol Oncol Res. https://doi.org/10.1007/s12253-019-00727-9(Epub ahead of print)
Travaglino A, Raffone A, Saccone G et al (2019) Nuclear expression of β-catenin in endometrial hyperplasia as marker of premalignancy. APMIS. https://doi.org/10.1111/apm.12988(Epub ahead of print)
Travaglino A, Raffone A, Saccone G et al (2019) Significant risk of occult cancer in complex non-atypical endometrial hyperplasia. Arch Gynecol Obstet. https://doi.org/10.1007/s00404-019-05299-2(Epub ahead of print)
Raffone A, Travaglino A, Mascolo M et al (2019) TCGA molecular groups of endometrial cancer: pooled data about prognosis. Gynecol Oncol 155(2):374–383
Hu S, Hinson JL, Matnani R, Cibull ML, Karabakhtsian RG (2018) Are the uterine serous carcinomas underdiagnosed? Histomorphologic and immunohistochemical correlates and clinical follow-up in high-grade endometrial carcinomas initially diagnosed as high-grade endometrioid carcinoma. Mod Pathol 31(2):358–364
Fadare O, Roma AA, Parkash V, Zheng W, Walavalkar V (2018) Does a p53 “wild-type” immunophenotype exclude a diagnosis of endometrial serous carcinoma? Adv Anat Pathol 25(1):61–70
Ritterhouse LL, Howitt BE (2016) Molecular pathology: predictive, prognostic, and diagnostic markers in uterine tumors. Surg Pathol Clin 9(3):405–426
Taskin OÇ, Onder S, Topuz S et al (2017) A selected immunohistochemical panel aids in differential diagnosis and prognostic stratification of subtypes of high-grade endometrial carcinoma: a clinicopathologic and immunohistochemical study at a single institution. Appl Immunohistochem Mol Morphol 25(10):696–702
Stewart CJ, Crook ML (2015) SWI/SNF complex deficiency and mismatch repair protein expression in undifferentiated and dedifferentiated endometrial carcinoma. Pathology 47(5):439–445
Rosa-Rosa JM, Leskelä S, Cristóbal-Lana E et al (2016) Molecular genetic heterogeneity in undifferentiated endometrial carcinomas. Mod Pathol 29(11):1390–1398
Espinosa I, Lee CH, D’Angelo E, Palacios J, Prat J (2017) Undifferentiated and dedifferentiated endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Am J Surg Pathol 41(8):1121–1128
Köbel M, Hoang LN, Tessier-Cloutier B et al (2018) Undifferentiated endometrial carcinomas show frequent loss of core switch/sucrose nonfermentable complex proteins. Am J Surg Pathol 42(1):76–83
Wortman BG, Bosse T, Nout RA et al (2018) Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: evaluation of the pilot phase of the PORTEC-4a trial. Gynecol Oncol 151(1):69–75
Soumerai TE, Donoghue MTA, Bandlamudi C et al (2018) Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin Cancer Res 24(23):5939–5947
Stelloo E, Bosse T, Nout RA et al (2015) Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 28(6):836–844
Murali R, Davidson B, Fadare O et al (2019) High-grade endometrial carcinomas: morphologic and immunohistochemical features, diagnostic challenges and recommendations. Int J Gynecol Pathol 38(Suppl 1):S40–S63
Singh N, Hirschowitz L, Zaino R et al (2019) Pathologic prognostic factors in endometrial carcinoma (other than tumor type and grade). Int J Gynecol Pathol 38(Suppl 1):S93–S113
Han KH, Kim HS, Lee M, Chung HH, Song YS (2017) Prognostic factors for tumor recurrence in endometrioid endometrial cancer stages IA and IB. Medicine (Baltimore) 96(21):e6976
Cuylan ZF, Oz M, Ozkan NT et al (2018) Prognostic factors and patterns of recurrence in lymphovascular space invasion positive women with stage IIIC endometriod endometrial cancer. J Obstet Gynaecol Res 44(6):1140–1149
Funding
No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
AT and AR independently assessed electronic search, eligibility of the studies, inclusion criteria, risk of bias, data extraction, and data analysis. CS, RE, PM, CG, and GO contributed to the elaboration of methods for risk of bias assessment, data extraction, and analysis. AT, AR, LI, and FZ conceived the study; AT, AR, CS, LI, and FZ worked on the design of the study; AT, AR, RE, PM, and CG worked on the manuscript preparation; LI and FZ supervised the whole study.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
404_2020_5542_MOESM1_ESM.tif
Flow diagram of studies identified in the systematic review (Prisma template [Preferred Reporting Item for Systematic Reviews and Meta-analyses]). (TIFF 19 kb)
404_2020_5542_MOESM2_ESM.tif
Risk of bias within studies. Upper graph: Summary of risk of bias for each study; Plus sign: low risk of bias; minus sign: high risk of bias; question mark: unclear risk of bias. Lower graph: Risk of bias graph about each risk of bias item presented as percentages across all included studies (TIFF 22 kb)
404_2020_5542_MOESM3_ESM.tif
Forest plots of hazard ratio (HR) for disease-specific survival in the TCGA molecular subgroups of endometrial carcinoma (“all-histotypes” group) (TIFF 203 kb)
404_2020_5542_MOESM4_ESM.tif
Forest plots of hazard ratio (HR) for progression-free survival in the TCGA molecular subgroups of endometrial carcinoma (“all-histotypes” group) (TIFF 204 kb)
404_2020_5542_MOESM5_ESM.tif
Forest plots of hazard ratio (HR) for disease-specific survival in the TCGA molecular subgroups of endometrial carcinoma (“endometrioid” group) (TIFF 155 kb)
404_2020_5542_MOESM6_ESM.tif
Forest plots of hazard ratio (HR) for progression-free survival in the TCGA molecular subgroups of endometrial carcinoma (“endometrioid” group) (TIFF 182 kb)
404_2020_5542_MOESM7_ESM.tif
Scale of hazard ratio for disease-specific survival (DSS) in the TCGA subgroups of endometrial carcinoma stratified by histotype: endometrioid (pink squares), non-endometrioid (blue squares) or all histotypes (no squares) (TIFF 65 kb)
404_2020_5542_MOESM8_ESM.tif
Scale of hazard ratio for progression-free survival (PFS) in the TCGA subgroups of endometrial carcinoma stratified by histotype: endometrioid (pink squares), non-endometrioid (blue squares) or all histotypes (no squares) (TIFF 66 kb)
Rights and permissions
About this article
Cite this article
Travaglino, A., Raffone, A., Stradella, C. et al. Impact of endometrial carcinoma histotype on the prognostic value of the TCGA molecular subgroups. Arch Gynecol Obstet 301, 1355–1363 (2020). https://doi.org/10.1007/s00404-020-05542-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05542-1