Abstract
Purpose
To evaluate the benefits of LT4 treatment on pregnancy outcomes in SCH women.
Study design
PubMed [including Medline], Web of Science, Wiley, Google Scholar, Science direct and Scopus were searched for identifying and retrieving all English articles published up to May 2018 on the effects of levothyroxine treatment on pregnancy outcomes in pregnant women with SCH compared to untreated or healthy controls. In this systematic review and meta-analysis, both fixed and random effect models were applied to estimate the pooled effect size. Heterogeneity and publication bias were evaluated using the I-squared (I2) and Begg’s statistics, respectively. We also explored heterogeneity sources using meta-regression models and sensitivity analysis.
Results
Data of 13 cohort studies and randomized controlled trials with a total of 11,503 participants were analyzed. This meta-analysis showed that pregnant women with SCH treated with levothyroxine had lower chances of pregnancy loss (OR 0.78, 95% CI 0.66–0.94; I2 = 0%) and higher chances for live birth rates (OR 2.72, 95% CI 1.44–5.11; I2 = 25%) than the placebo group. Compared to euthyroid women, SCH patients treated with levothyroxine had higher odds ratio for preterm labor (OR 1.82, 95% CI 1.14–2.91; I2 = 0%).
Conclusions
Results of this study showed that the effects of treatment with levothyroxine in SCH pregnant women are not the same for all pregnancy outcomes. Levothyroxine treatment in these patients can reduce pregnancy loss. Considering the limited number of studies available, further studies are warranted to document more precise data on other consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subclinical hypothyroidism (SCH) is defined as elevated thyroid stimulating hormones (TSH) with normal levels of free triiodothyronine (FT3) and free thyroxine (FT4) [1]. It is the most common thyroid dysfunction during pregnancy, with its prevalence varying between 4 and 13%, based on different cutoff values for TSH, various ethnicities, iodine consumption and nutritional life style as well as study designs [2, 3].
Several observational studies demonstrate the association between subclinical hypothyroidism during pregnancy and adverse pregnancy outcomes, including pregnancy loss, gestational diabetes, gestational hypertension, preeclampsia, intrauterine growth restriction, placental abruption, premature rupture of membranes, preterm delivery, low birth weight, small for gestational age, low Apgar score, fetal and neonatal distress, neonatal death, and decreased IQ in the offspring [4,5,6,7,8]. Although thyroid peroxidase antibody (TPOAb) status has not been reported in the majority of these investigations, prospective studies suggest higher rates of pregnancy complications in both SCH-TPOAb+ and SCH-TPOAb− pregnant women [8, 9]. There were some studies that retrospectively compared the pregnancy outcomes of women with SCH who received LT4 with those of women not receiving it, and reported a possible beneficial effect for LT4, in terms of pregnancy outcomes [10]. Additionally, there was only one randomized clinical trial that investigated the effect of levothyroxine (LT4) in TPOAb− pregnant women with subclinical hypothyroidism [11], and the rest were conducted among TPOAb+ women [9, 12] or both TPOAb+ and TPOAb− subjects [13, 14]. There were several meta-analyses, mainly based on observational studies, reporting a potential additional risk of adverse pregnancy outcomes in SCH pregnant women [1, 15,16,17] that basically focus on the adverse pregnancy outcomes rather than the benefits of receiving LT4. One meta-analysis assessing the beneficial effect of LT4 on pregnancy outcomes of infertile women, based on three available trials, reported a significantly lower rate of miscarriage in women who received LT4 [18], results, however, not generalized to the whole population.
Despite several guidelines developed by the American Thyroid Association (ATA) [19], The European Thyroid Association [20] and the Endocrine Society (ES) [21], and the universal consensus on adverse effects of overt hypothyroidism on pregnancy outcomes, and the strong recommendation for LT4 treatment of these women during pregnancy, no consensus has yet been reached on whether or not to treat subclinical hypothyroid (SCH) women for improving pregnancy outcomes, and the uncertainty regarding the prescription of levothyroxine therapy in preventing the adverse outcomes still remains [20].
The aim of the current systematic review and meta-analysis was mainly to assess the effect of levothyroxine treatment on pregnancy outcome in women with subclinical hypothyroidism.
Materials and methods
This systematic review and meta-analysis was designed according to the reporting items for systematic reviews and meta-analyses referred to in the PRISMA statement to assess the following objectives:
-
1.
To assess the odds ratios of adverse pregnancy outcomes in subclinical hypothyroid women treated with levothyroxine compared to untreated controls.
-
2.
To assess the odds ratios of adverse pregnancy outcomes in subclinical hypothyroid women treated with levothyroxine compared to healthy controls.
Search strategy
A comprehensive electronic literature search was performed in PubMed [including Medline], Web of Science, Wiley, Google Scholar, Science direct and Scopus databases, for retrieving English articles published from inception to May 2018 regarding the effects of levothyroxine treatment on pregnancy outcomes in pregnant women with subclinical hypothyroidism, compared to untreated or healthy controls.
Before initiation of the study, searches were conducted independently by the authors to determine the eligibility criteria. First, the search in Pubmed was performed, based on MESH using the following keywords (alone or in combination): (“levothyroxine” OR “LT4” OR “thyroxine supplementation”) AND (“subclinical hypothyroidism” OR “SCH”) AND (“pregnancy outcomes” OR “abortion” OR “miscarriage “OR “pregnancy loss” OR “fetal death” OR “stillbirth” OR “preeclampsia” OR “gestational hypertension” OR “PIH” OR “gestational diabetes” OR “GDM” OR “hemorrhage” OR “postpartum hemorrhage” OR “PPH” OR “placenta abruption” OR “placenta previa” OR “preterm” OR “prematurity” OR “premature rupture of membrane” OR “PROM” OR “intrauterine growth restriction” OR “IUGR” OR “small for gestational age” OR “low birth weight” OR “LBW” OR “oligohydramnios” OR “apgar” OR “fetal distress” OR “neonatal distress” OR “RDS” OR “neonatal death” OR “neonatal admission” OR “NICU admission” OR “malformation”). Search strategy was almost similar for all databases. Search limitations were humans and English language. Searches were conducted based on ‘all fields’ in the PubMed and on ‘titles, abstracts and keywords’ in other databases. In addition, the reference lists of included studies were reviewed to retrieve additional publications that could be used in this review.
Eligibility criteria
We searched studies restricted to English published papers from inception to May 2018. Studies were eligible if they had: (1) a study population including pregnant women with subclinical hypothyroidism diagnosed by any criteria, who had been treated with levothyroxine; (2) assessed at least one pregnancy outcome, including pregnancy loss (abortion, fetal death or stillbirth), hypertension, preeclampsia, gestational diabetes, antenatal or postpartum hemorrhage, placenta abruption, placenta previa, preterm, premature rupture of membrane (PROM), Intrauterine growth restriction (IUGR), low birth weight (LBW), small for gestational age, fetal or neonatal distress, low Apgar score, neonatal death, malformation, neonatal admission or NICU admission.
We also excluded: (1) non-human studies, reviews, commentaries, editorials, letters, meeting abstracts, case reports and cross-sectional and case–control studies; (2) studies that had a population with only overt hypothyroidism or hyperthyroidism; (3) studies that did not provide accurate and clear data or methods and (4) studies without a control group.
Study selection
All relevant RCTs or cohort studies assessing the effect of LT4 therapy compared to placebo or no treatment on pregnancy outcomes of pregnant women with subclinical hypothyroidism were selected for the meta-analysis. At least one of the pregnancy outcomes mentioned above had to be reported.
We screened the search results based on pre-defined eligibility criteria. Initial selection was performed based on study titles. After deleting duplicates, we reviewed the abstracts of all remaining records. Any disagreement in the selection of abstracts was resolved by consensus or by another reviewer (FRT). Full-text articles of all selected abstracts were obtained for reviewing and data processing.
Data extraction
Two reviewers performed data extraction from full texts and checked the data extracted to eliminate errors. For each study, the following information was extracted: authors, year of publication, study design, intervention, characteristics of study population and outcome assessment. To prevent extraction errors, all reviewers performed a quality control check between the final data used in the meta-analysis and the original publications.
Quality assessment
Two reviewers, who were blinded to study author, institution, journal name, volume and page, assessed the quality of each study separately. Disagreement was resolved by other reviewers.
All the studies used in this meta-analysis were critically evaluated using the modification of the Newcastle–Ottawa Quality Assessment Scale (NOS) of nonrandomized studies [22] for cohort studies, and the CONSORT 2010 checklist [23] for randomized controlled trial studies (RCTs).
The CONSORT 2010 checklist provides guidance for reporting all randomized controlled trials. We used the method and results section to evaluate the quality of RCTs.
Studies with scores > 70% of the highest level of CONSORT checklist or NOS were considered as having good-, 40–70% as fair-, and < 40% as poor quality.
Risk of bias assessment
Risk of bias for each included study was assessed using the Cochrane Collaboration’s tools; these tools were designed for various methodological studies including RCTs and observational studies such as cohorts.
Six domains related to risk of bias were assessed for each RCT, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.
For cohort studies, seven domains of risk of bias were assessed including selection of exposed and non-exposed cohorts, the assessment of exposure, presence of the outcome of interest at initiation of study, control of prognostic variables, assessment of the presence or absence of prognostic factors, outcome assessment, and adequacy of the cohorts’ follow-ups.
Judgments of the review authors regarding bias were categorized as “low risk”, “high risk”, and “unclear risk’’ of bias for RCTs, and as “definitely No (low risk of bias)”, “probably no”, “definitely yes (high risk of bias)” and “probably Yes” for cohort studies.
Outcome measures
Maternal, fetal and neonatal outcomes of interest were categorized into five composite outcomes, including (1) pregnancy loss (abortion, fetal death and still birth), (2) adverse maternal complications (gestational hypertension, preeclampsia and gestational diabetes), (3) obstetrical hemorrhage (antenatal hemorrhage, postpartum hemorrhage, placenta abruption, and placenta previa), (4) preterm/LBW (preterm labor, PROM, IUGR and LBW) oligohydramnios, and (5) adverse neonatal complications (fetal distress, RDS, retinopathy, low Apgar score, neonatal death, malformation, neonatal admission, and NICU admission).
Statistical analysis
The Software package STATA (version 12; STATA Inc., College Station, TX, USA) was used to conduct statistical analysis. Meta-analysis was performed to evaluate the pooled ORs of our outcomes of interest. Forest plots demonstrate the weighted ORs of outcomes.
Heterogeneity was evaluated by the I-squared index, with values over 50% interpreted as heterogeneity. Publication bias was assessed by Begg’s test, and p values < 0.05 were considered as significant, using the Trim and fill method to adjust bias. Meta-analysis through the Mantel–Haenszel pooling method was used to estimate the pooled mean of age, BMI and OR of thyroid dysfunction in subgroups of the control group (Euthyroid and placebo). Meta-regression models were performed to estimate the pooled OR of adverse pregnancy outcomes based on the TSH cutoff value (2.5 mIU/L vs. > 2.5 mIU/L) and initiation time of treatment (before vs. during pregnancy). To explore heterogeneity due to the influence of TPOAb status on the results, we conducted a sensitivity analysis on the main outcomes.
Results
Search results and study selection
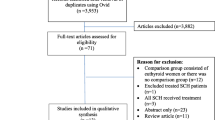
Figure 1 illustrates a flowchart of the literature search and its results. Of the initial 73 references that were identified, 25 duplicates were removed (i.e., identical articles retrieved from multiple search strategies). In the first step, selection was based on titles and abstracts from the retrieved references. According to the selection criteria, 23 articles were identified for further full-text assessment. Finally, 13 full-text articles were included for the meta-analysis.
Quality assessment and risk of bias
Results of quality assessment showed that 8 studies were classified as good and 5 as fair quality. No study had poor quality (Table 1; Supplementary Tables 1, 2).
Supplementary Figs. 1 and 2 show the details of risk of bias in the published studies. Most cohort studies had a low risk of bias for selection (86%), assessment of exposure (100%), assessment of presence of outcome of interest at initiation (100%), assessment of outcome (100%) and follow up (100%); 57% of studies had a high risk of bias for match exposed and unexposed and 43% for assessment of the presence of prognostic factors, 14% of studies had a high risk of bias for selection. Fourteen percent of studies were probable high risk of bias for match exposed and unexposed and assessment of the presence of prognostic factors (Supplementary Fig. 1).
Most RCT studies were at a low risk of bias for random sequence generation (83%), blinding of outcome assessment (100%), incomplete outcome data (100%) and selective outcome reporting (100%); 66% of these studies were at high risk of bias for blinding of participants and some biases were probable, like blinding of incomplete outcome data. Most studies had a low risk of bias (Supplementary Fig. 2).
Generally, most studies had acceptable validity (low risk of bias), indicating the acceptable quality of these studies in most aspects.
Study characteristics
Overall, we found 13 articles that met our eligibility criteria. Characteristics of the selected studies are presented in Table 1. Among these, six were RCTs and seven were cohorts. The studies were published between 2010 and 2018. In most studies, the cutoff value for TSH was 2.5 mIU/L, and only in four studies it was > 2.5 mIU/L [13, 24,25,26]. All studies had treatment and placebo or no treatment control groups. In four studies [11, 12, 27, 28], in addition to the placebo control or the group without treatment, there was a euthyroidism control group (Table 1).
The pooled mean age and BMI (95% CI) were 29.87 (29.77–29.96) years and 24.57 (24.44–24.70) kg/m2, respectively.
Meta-analysis of outcomes
Table 2 presents the results of heterogeneity, pooled OR and publication bias of maternal, fetal and neonatal composite outcomes in SCH pregnant women treated with levothyroxine in comparison with the placebo- or euthyroid controls. SCH pregnant women treated with levothyroxine had a lower pooled ORs for outcomes related to pregnancy loss compared to those treated with placebo (OR 0.78, 95% CI 0.66–0.94; I2 = 0%) (Fig. 2; Table 2). There were no significant differences in pooled ORs of adverse maternal complications, obstetrical hemorrhage, preterm/LBW/oligohydramnios and adverse neonatal complications, between patients treated with levothyroxine and the placebo group (Table 2; Fig. 3). Results of meta-analysis on separate outcomes showed that SCH pregnant women treated with levothyroxine had a lower pooled OR for abortion (OR 0.37, 95% CI 0.18–0.77; I2 = 25%) and a higher pooled OR for live birth rate (OR 2.72, 95% CI 1.44–5.11; I2 = 25%), compared to those treated with placebo (Table 3; Fig. 4). The pooled OR of preterm labor and neonatal/NICU admission in SCH pregnant women treated with levothyroxine had no statistically significant differences compared to the placebo group (Table 3; Fig. 4).
We observed that SCH pregnant women treated with levothyroxine had a lower pooled OR for adverse neonatal complications than euthyroid women (OR 0.59, 95% CI 0.36–0.99; I2 = 0%). Our results also showed a higher pooled ORs for preterm/LBW/oligohydramnios outcomes in SCH pregnant patients treated with levothyroxine compared to euthyroid pregnant women (OR 1.74, 95% CI 1.15–2.65; I2 = 0%) (Table 2; Fig. 3). There were no significant differences in the pooled ORs of other outcomes (adverse maternal complication, obstetrical hemorrhage, and preterm/LBW/oligohydramnios) between pregnant women treated with levothyroxine and those who were euthyroid (Table 2). The pooled OR of preterm birth in pregnant women treated with levothyroxine was higher than euthyroid women (OR 1.82, 95% CI 1.14–2.91; I2 = 0%). The pooled ORs of abortion, live birth rate and neonatal/NICU admission in SCH pregnant patients treated with levothyroxine showed no significant differences compared to euthyroid controls (Table 3).
Risk of bias assessment
Supplementary files 1 and 2 show the results of risk of bias in the published studies. Most RCT studies were at a low risk of bias of random sequence generation, blinding of participants and personnel, blinding of outcome, incomplete outcome data and selective outcome reporting; however, in these studies, some biases were more probable, such as blinding of participants and personnel.
Although cohort studies were at a low risk of bias for selection, assessment of exposure, the presence of the outcome of interest at initiation of study, outcome assessment and adequacy of follow-up duration, some of them had serious risk of bias in the control of prognostic variables.
Since most studies had appropriate validity (low risk of bias), the quality of these studies was acceptable in most aspects.
Meta-regression analysis base on TSH-cut and initiation time of treatment
Results of meta-regression based on the TSH cutoff value did not show statistically significant difference in the pooled ORs of adverse pregnancy outcomes with TSH cutoff 2.5 mIU/L compared to those with cutoff > 2.5 mIU/L (Supplementary Table 11). In addition, meta-regression based on the initiation time of treatment demonstrated that there was no statistically significant difference in the pooled ORs of adverse pregnancy outcomes (abortion, and pregnancy loss) between women treated before pregnancy and those treated during pregnancy (Supplementary Table 12).
Sensitivity analysis based on TPOAb status
Results of sensitivity analysis, presented in a supplementary file (supplementary figures and Tables 3–10), show that there were no significant differences in pooled ORs of adverse pregnancy outcomes after excluding studies with different TPOAb status.
Discussion
Despite the well-documented beneficial effect of levothyroxine therapy in pregnant women with overt hypothyroidism, there is insufficient evidence to clarify the effects of LT4 treatment on maternal, fetal and neonatal outcomes in patients with SCH. This meta-analysis showed that SCH pregnant women treated with levothyroxine had lower chances for pregnancy loss and higher chances for live birth rate than the placebo group, findings which need to be interpreted with caution, due to the inadequate number of randomized clinical trials or enough power to eliminate the risks attributed to thyroid autoimmunity among these SCH pregnant women. We did not find any significant difference in the chances of adverse maternal complication, obstetrical hemorrhage and neonatal/NICU admission between SCH patients treated with levothyroxine and control groups (placebo and euthyroid).
The underlying mechanisms involved in developing adverse pregnancy outcomes in SCH, especially in those without thyroid auto immunity, have not been completely elucidated [12]. It has been documented that thyroid autoimmunity may increase the risk of preterm birth through both TSH and non-TSH-dependent mechanisms. Baseline TSH levels of pregnant women were higher in TPOAb+ women than their TPOAb− counterparts, in whom these levels were well within the euthyroid range [29], as a result, of which they had a subtle preexisting thyroid dysfunction that could possibly worsen during pregnancy and they may not be able to respond adequately to the increased demand for synthesis of thyroid hormones during pregnancy [30]. On the other hand, pregnancy is an inflammatory process, involving a shift in the regulation of cytokine networks within the local placental–decidual environment or fetal development [31] which can be associated with a direct destructive effect on the placenta. Therefore, dysregulation of local inflammatory processes in TPO-Ab+ women may be associated with premature delivery [32]. However, data on the relationship between subclinical thyroid dysfunction and/or thyroid autoimmunity with preterm labor are inconsistent, and a recent meta-analysis including 14 cohort and one case–control studies demonstrated no significant increase in the OR of preterm labor in women with subclinical hypothyroidism [33]. Additionally despite the assumed impact of LT4 on appropriate placentation, a recent large randomized clinical trial of preconception LT4 treatment in TPO-Ab+ infertile women with TSH < 5 mIU/L revealed no benefits on miscarriage, live birth or preterm delivery rates [34].
Hypothyroidism is an independent risk factor for cardiovascular diseases [35]. Despite the theoretical relationship of thyroid disorders with cardio-metabolic outcomes, our study did not detect any significant difference in the chances of SCH pregnant women, treated with levothyroxine developing gestational hypertension, preeclampsia and gestational diabetes and the placebo or euthyroid controls.
Our study showed a reduced OR of miscarriage in pregnant women who received LT4 compared to the placebo group, a finding in agreement with a meta-analysis conducted by Maraka et al. [1] who reported that pregnant women with untreated SCH had a higher risk for pregnancy loss and neonatal death, compared to euthyroid women. Velkeniers et al. [18] study also demonstrated that levothyroxine supplementation vs. placebo/no treatment was associated with a significant increase in delivery rate and decrease in miscarriage among those who had used assisted reproduction technologies (ART). Zhang et al. [17] meta-analysis revealed that compared to euthyroid pregnant women, patients with untreated SCH had a higher prevalence of miscarriage before 20 weeks of pregnancy, and they also found that the prevalence of miscarriage in patients not treated with LT4 significantly increased, compared to those who were. A recent meta-analysis by Rao et al. [36] including 8 randomized controlled trials and 5 retrospective studies demonstrated the beneficial effects of LT4 supplementation on the reduced risk of pregnancy loss but not preterm birth, among pregnant women with SCH and/or thyroid autoimmunity.
Some differences in the results of various meta-analyses could be attributed to their different eligibility criteria. Rao et al.’s study [36] assessed pregnant women with SCH and thyroid autoimmunity, whereas our meta-analysis was limited to studies conducted on pregnant women with SCH, regardless of their positive or negative TPOAb. Also, Zhang et al. meta-analysis [17] included only cohort studies, while our met-analysis included both RCT and cohort studies.
Several observational studies have reported a link between SCH and increased risk of pregnancy loss [10,11,12, 24, 25, 28], maternal complications such as gestational hypertension, preeclampsia and diabetes [6, 37], obstetrical hemorrhage [38], preterm birth/LBW [28] and adverse neonatal outcomes [39]. However, there is strong evidence indicating that thyroid autoantibodies, as the most common cause of SCH, may directly exert their adverse effects on maternal, fetal and neonatal outcomes [40]; as a result, treatment of these women with LT4 may not completely resolve these adverse effects on pregnancy outcomes. It has been shown that thyroid autoantibodies may adversely affect pregnancy through changes in the profiles of endometrial T cells, hyperactivity and increased migration of cytotoxic natural killer cells, affecting zonapellucida, human chorionic gonadotropin receptors and other placental antigens [41], which can to some extent explain the higher prevalence of preterm/LBW/oligohydramnios outcomes in pregnant patients with treated SCH compared to euthyroid pregnant women, even after treatment with levothyroxine in the present meta-analysis.
We also observed that SCH patients treated with levothyroxine had no significant differences in the OR of obstetrical hemorrhage compared to placebo or euthyroid controls. Although Maraka et al. [1] observed a higher risk for obstetrical hemorrhage in pregnant women with untreated SCH compared to euthyroid women; their study showed that treated patients had no lower risk compared to the placebo or euthyroid control groups, suggesting that levothyroxine therapy was not effective in preventing obstetrical hemorrhage.
While this study could not detect any difference in the ORs of adverse neonatal complications (fetal distress, RDS, retinopathy, low Apgar score, neonatal death, malformation, neonatal admission, and NICU admission) in SCH pregnant women treated with levothyroxine compared to placebo, the OR was lower in treated subjects compared to euthyroid ones. This contradictory finding suggests that the lower OR of neonatal outcomes in patients diagnosed with SCH may be due to an increase in their medical visits and, consequently, an increase in their maternity care. However, when we considered neonatal/NICU admission as a separate outcome, the OR of this outcome had no difference in SCH pregnant women treated with levothyroxine in comparison to placebo or euthyroid controls.
While maternal thyroid hormones are essential for the fetal development before the second trimester, before when the thyroid gland becomes functional, in some cases this treatment was begun after 12 weeks of gestation [42]. A limited number of studies have been assessed the timing effect on levothyroxine treatment against SCH with inconclusive results [13, 42]. Zhao et al.’s cohort study [42] showed that administration of LT4 initiated in the first trimester was associated with decreased risk of adverse pregnancy outcomes compared to those treated in the second trimester, suggesting that timing plays an important role in the prescription of levothyroxine to reduce pregnancy complications in SCH. One clinical trial reported that hormone therapy could not improve the adverse obstetrics outcomes in SCH pregnant women whose treatment was initiated at an average of 17 weeks of gestation. In our meta-analysis, the initiation of levothyroxine administration was different [initiated before pregnancy, during pregnancy, or not reporting timing]. However, meta-regression based on the initiation time of treatment (before or after pregnancy) was not significant, indicating that the time frame at least in terms of initiation before or after pregnancy had no significant effects on reducing adverse pregnancy outcomes (Supplementary Table 12).
The main strength of the current meta-analysis compared to others was including only the studies that assessed the effect of levothyroxine in pregnant women with SCH. We also evaluated the impact of LT4 treatment on various pregnancy outcomes in comparison to both SCH pregnant women not receiving LT4, and also, euthyroid pregnant women. The study, however, has its limitations that should also be considered when interpreting the findings. First, there is no universal cutoff value for TSH in the diagnosis of SCH. In 2011, the initial recommendations by the American Thyroid Association (ATA) advocated the use of a laboratory or population-based pregnancy-specific TSH reference range; if unavailable a TSH upper limit of 2.5, 3.0 and 3.0 mIU/L in the 1st, 2nd and 3rd trimesters can be used, respectively [43]. This guideline was revised in 2017, suggesting a TSH cutoff value of > 4mIU/L in the first trimester [19]. Applying the pre-defined cut points may result in overtreatment of euthyroid pregnant women despite lack of sufficient evidence. Considering the heterogeneity of results derived from diverse studies with different thresholds for TSH [13, 24, 25, 28, 34], we conducted a meta-regression based on the TSH cutoff value; however, it made no significant difference in our results (Supplementary Table 11). Therefore, the TSH cutoff was not a source of heterogeneity. Second, TPOAb status was not determined in all studies included; some of the studies were conducted on both TPOAb+ and TPOAb− participants. To assess the heterogeneity caused by the influence of TPOAb status on our results, we performed a sensitivity analysis, which showed no significant differences in our results.
Other limitations were the lack of: (1) adequate well-designed randomized clinical trials, (2) an equal dose of LT4 for treatment and (3) adequate power for subgroup analysis according to the type of study or gestational age for initiation of treatment. Needless to say, further well-designed clinical trials are required to assess the effect of levothyroxine on adverse pregnancy outcomes in women with SCH.
Conclusion
Despite observing lower chances for pregnancy loss and higher chances for live birth rate in SCH pregnant women who received LT4 treatment during pregnancy, these findings are not mainly based on randomized clinical trials and need to be interpreted with caution. More well-designed randomized clinical trials, considering thyroid autoimmunity of participants, using a similar threshold of TSH for making a precise diagnosis and universal dose for treatment initiated at early pregnancy or even preferably at the preconception period, are vital for making evidence-based decisions regarding LT4 treatment of SCH pregnant women. Till then, physicians should consider the potential benefits of levothyroxine treatment, taking into account the preferences and conditions of these pregnant women.
References
Maraka S, Ospina NM, O’Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC 3rd, Stan MN, Murad MH, Montori VM (2016) Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 26(4):580–590
Al Shanqeeti SA, Alkhudairy YN, Alabdulwahed AA, Ahmed AE, Al-Adham MS, Mahmood NM (2018) Prevalence of subclinical hypothyroidism in pregnancy in Saudi Arabia. Saudi Med J 39(3):254–260
Garmendia Madariaga A, Santos Palacios S, Guillen-Grima F, Galofre JC (2014) The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab 99(3):923–931
Chen LM, Du WJ, Dai J, Zhang Q, Si GX, Yang H, Ye EL, Chen QS, Yu LC, Zhang C, Lu XM (2014) Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PLoS One 9(10):e109364
Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB (2011) Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 96(10):3234–3241
Tudela CM, Casey BM, McIntire DD, Cunningham FG (2012) Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 119(5):983–988
Wang S, Teng WP, Li JX, Wang WW, Shan ZY (2012) Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest 35(3):322–325
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A (2010) Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 95 (9):E44–E48. https://academic.oup.com/jcem/article/95/9/E44/2835150
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A (2010) Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab 95(4):1699–1707
Maraka S, Singh Ospina NM, O’Keeffe DT, Rodriguez-Gutierrez R, Espinosa De Ycaza AE, Wi CI, Juhn YJ, Coddington CC 3rd, Montori VM, Stan MN (2016) Effects of levothyroxine therapy on pregnancy outcomes in women with subclinical hypothyroidism. Thyroid 26(7):980–986
Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Minooee S, Rahmati M, Azizi F (2018) Effects of levothyroxine on pregnant women with subclinical hypothyroidism, negative for thyroid peroxidase antibodies. J Clin Endocrinol Metab 103(3):926–935
Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Alavi Majd H, Azizi F (2017) Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 176(2):253–265
Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, Reddy UM, Wapner RJ, Thorp JM Jr., Saade G, Tita AT, Rouse DJ, Sibai B, Iams JD, Mercer BM, Tolosa J, Caritis SN, VanDorsten JP, Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N (2017) Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 376(9):815–825
Ma L, Qi H, Chai X, Jiang F, Mao S, Liu J, Zhang S, Lian X, Sun X, Wang D, Ren J, Yan Q (2016) The effects of screening and intervention of subclinical hypothyroidism on pregnancy outcomes: a prospective multicenter single-blind, randomized, controlled study of thyroid function screening test during pregnancy. J Matern Fetal Neonatal Med 29(9):1391–1394
Nasirkandy MP, Badfar G, Shohani M, Rahmati S, YektaKooshali MH, Abbasalizadeh S, Soleymani A, Azami M (2017) The relation of maternal hypothyroidism and hypothyroxinemia during pregnancy on preterm birth: an updated systematic review and meta-analysis. Int J Reprod Biomed 15(9):543–552
Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, Papadimas I (2010) Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol 162(4):643–652
Zhang Y, Wang H, Pan X, Teng W, Shan Z (2017) Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: a systematic review and meta-analysis. PLoS One 12(4):e0175708
Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P (2013) Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta-analysis of RCTs. Hum Reprod Update 19(3):251–258
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S (2017) 2017 guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389
Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B (2014) 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 3(2):76–94
De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S (2012) Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97(8):2543–2565
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 8(1):18
Abdel Rahman AH, Aly Abbassy H, Abbassy AA (2010) Improved in vitro fertilization outcomes after treatment of subclinical hypothyroidism in infertile women. Endocr Pract 16(5):792–797
Cai Y, Zhong L, Guan J, Guo R, Niu B, Ma Y, Su H (2017) Outcome of in vitro fertilization in women with subclinical hypothyroidism. Reprod Biol Endocrinol 15(1):39
Kim CH, Ahn JW, Kang SP, Kim SH, Chae HD, Kang BM (2011) Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 95(5):1650–1654
Al-Anbari L A (2017) Thyroxine Supplementation Improve Intrauterine Insemination Outcome in patients with subclinical Hypothyroidism. J Pharm Sci Res 9(10):1768–1772. https://www.jpsr.pharmainfo.in/Documents/Volumes/vol9Issue10/jpsr09101725.pdf
Bernardi LA, Cohen RN, Stephenson MD (2013) Impact of subclinical hypothyroidism in women with recurrent early pregnancy loss. Fertil Steril 100(5):1326–1331
Pearce E, Oken E, Gillman M, Lee S, Magnani B, Platek D, Braverman L (2008) Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocrine Pract 14(1):33–39
Banerjee S (2011) Thyroid disorders in pregnancy. J Assoc Physicians India 59(Suppl):32–34
Weetman AP (2010) Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol 6(6):311–318
Sailakshmi M, Rekha B, Akash SS (2014) Autoimmune thyroid disease in pregnancy. Int J Reprod Contracept Obstetr Gynecol 3(2):321–324
Sheehan PM, Nankervis A, Araujo Junior E, Da Silva Costa F (2015) Maternal thyroid disease and preterm birth: systematic review and meta-analysis. J Clin Endocrinol Metab 100(11):4325–4331
Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, Li R, Liu P, Wang C, Tian Q, Zhou Z, Yang J, Liu Y, Wei R, Mol BWJ, Hong T, Qiao J (2017) Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA 318(22):2190–2198
Khatiwada S, Sah SK, Kc R, Baral N, Lamsal M (2016) Thyroid dysfunction in metabolic syndrome patients and its relationship with components of metabolic syndrome. Clin Diabetes Endocrinol 2(1):3
Rao M, Zeng Z, Zhou F, Wang H, Liu J, Wang R, Wen Y, Yang Z, Su C, Su Z, Zhao S, Tang L (2019) Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: a systematic review and meta-analysis. Hum Reprod Update 25(3):344–361
Duan Y, Peng W, Wang X, Tang W, Liu X, Xu S, Mao X, Feng S, Feng Y, Qin Y, Xu K, Liu C, Liu C (2009) Community-based study of the association of subclinical thyroid dysfunction with blood pressure. Endocrine 35(2):136–142
Breathnach FM, Donnelly J, Cooley SM, Geary M, Malone FD (2013) Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Aust N Z J Obstet Gynaecol 53(6):553–560
Debieve F, Duliere S, Bernard P, Hubinont C, De Nayer P, Daumerie C (2009) To treat or not to treat euthyroid autoimmune disorder during pregnancy? Gynecol Obstet Invest 67(3):178–182
He X, Wang P, Wang Z, He X, Xu D, Wang B (2012) Thyroid antibodies and risk of preterm delivery: a meta-analysis of prospective cohort studies. Eur J Endocrinol 167(4):455–464
Triggianese P, Perricone C, Conigliaro P, Chimenti MS, Perricone R, De Carolis C (2016) Peripheral blood natural killer cells and mild thyroid abnormalities in women with reproductive failure. Int J Immunopathol Pharmacol 29(1):65–75
Zhao L, Jiang G, Tian X, Zhang X, Zhu T, Chen B, Wang Y, Ma Q (2018) Initiation timing effect of levothyroxine treatment on subclinical hypothyroidism in pregnancy. Gynecol Endocrinol 34(10):845–848
Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W, American Thyroid Association Taskforce on Thyroid Disease During P, Postpartum (2011) Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21(10):1081–1125
Acknowledgements
The authors wish to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of the manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
SN was involved in study design, search in databases, studies selection, data analysis, manuscript drafting, and submitting manuscript. FRT was involved in study design, data analysis, manuscript drafting and critical discussions. MA contributed in data analysis, and critical discussion, execution and manuscript drafting. RBY contributed in statistical analysis, interpreting data and manuscript drafting. FA was involved in data analysis, manuscript drafting, revising and critical discussion.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This systematic review and meta-analysis study was approved by the ethics committee of the Research Institute of Endocrine Sciences (RIES) (approval no.: IR.SBMU.ENDOCRINE.REC.1397.126).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nazarpour, S., Ramezani Tehrani, F., Amiri, M. et al. Levothyroxine treatment and pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. Arch Gynecol Obstet 300, 805–819 (2019). https://doi.org/10.1007/s00404-019-05245-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05245-2