Abstract
Purpose
We aimed to perform a systematic review and meta-analysis addressing the efficacy of levothyroxine therapy in pregnant women with subclinical hypothyroidism considering most recent evidence and subgroups of interest for clinical practice.
Methods
PubMed, Embase, and Cochrane Central were searched from inception for randomized controlled trials (RCTs) comparing levothyroxine with placebo or no intervention in pregnant women with subclinical hypothyroidism. We used a random-effects model and conducted subgroup analyses based on thyroid peroxidase antibody status, thyroid stimulating hormone levels, fertility treatment, and recurrent miscarriage.
Results
We included 11 RCTs comprising 2,749 pregnant women with subclinical hypothyroidism. Patients treated with levothyroxine (1,439; 52.3%) had significantly lower risk of pregnancy loss (risk ratio 0.69; 95% confidence interval 0.52–0.91; p < 0.01; 6 studies). However, there was no significant association between levothyroxine and live birth (risk ratio 1.01; 95% confidence interval 0.99–1.03; p = 0.29; 8 studies). No statistically significant interaction was observed across subgroups (p > 0.05).
Conclusion
Levothyroxine replacement therapy for subclinical hypothyroidism during pregnancy may decrease pregnancy loss when early prescribed. Nevertheless, further investigation is needed in patients with thyroid stimulating hormone above four milliunits per liter, especially when associated with recurrent miscarriage or infertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study investigated the potential benefit of levothyroxine in patients with subclinical hypothyroidism during pregnancy considering different subgroups of clinical interest. While levothyroxine may decrease the risk of pregnancy loss, additional studies are required to investigate this treatment effect in patients with a history of recurrent miscarriage or infertility, particularly in cases where thyroid-stimulating hormone levels exceed four milliunits per liter. |
Introduction
Subclinical hypothyroidism (SCH) is a condition characterized by elevated serum thyroid-stimulating hormone (TSH) levels in the setting of normal serum thyroid hormone levels and the presence or absence of symptoms [1]. SCH is considered the most common thyroid disorder in pregnant women, with an estimated prevalence between 2.3 and 13.5% [2,3,4,5,6]. Despite its substantial prevalence and several studies suggesting an association with adverse maternal–fetal outcomes [7, 8], there is still controversy on whether medically managing pregnant women with SCH is appropriate. This can be attributed to the fact that the diagnostic criteria for SCH in pregnancy has evolved over time, and consequently, the literature contains divergent findings due to the un-uniformity of TSH cutoff values used for the diagnosis of SCH.
Given the geographic and ethnic variability in normal TSH concentrations during pregnancy, a reference limit of 4 milliunits per liter (mU/L) has been recommended by the 2017 American Thyroid Association (ATA) guidelines as a diagnostic criteria for SCH in the first trimester [9], which updated a previous recommendation of a limit of 2.5 mU/L from the 2011 guidelines [10]. At present, only two meta-analyses adopted the new 2017 ATA criteria and have shown no significant improvement with the use of levothyroxine therapy for pregnant women when analyzing only randomized controlled trials (RCTs) [11, 12]. However, these meta-analyses applied only one TSH levels criteria and had an overly narrow eligibility criteria, which may have led to less statistically powered conclusions. Moreover, additional RCTs have recently been published ever since, significantly increasing the pooled population and the statistical power that may result from further analyses.
Therefore, we performed an updated systematic review and meta-analysis of RCTs evaluating the role of levothyroxine in the treatment for SCH during pregnancy. Of note, we aimed to carry out a more inclusive analysis using both definition of SCH, and to explore the role of baseline characteristics on the efficacy of levothyroxine therapy in this population.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we aimed to assess pregnant women diagnosed with SCH (population) who were allocated to levothyroxine (intervention) or no levothyroxine (placebo or no intervention—control) and evaluate the results of pregnancy loss, live birth, and preterm birth before 37 weeks (outcomes) among RCTs (type of study) that followed patients until delivery (time). We included studies regardless of the selected ATA definition or ethnicity. Trials composed by euthyroid control, patients with thyroid diseases other than SCH, or overlapping populations, defined as studies recruiting from the same institution over an overlapping period, and screening studies, defined as trials that allocated patients to thyroid screening versus no screening and treated only those who presented with abnormal TSH levels, were excluded.
Two authors (H.P. and H.C.M.) systematically searched PubMed, Embase, and Cochrane Central from inception to February 1, 2023. The following terms with their respective mesh terms were used without filters, publication date, or language restrictions: (levothyroxine OR LT4 OR “thyroxine supplementation”) AND (“subclinical hypothyroidism” OR SCH) AND (pregnancy OR pregnant). The references from all included studies, previous systematic reviews and meta-analyses were also searched manually for any additional studies. Eventual conflicts were resolved by consensus among the authors. Two authors (G.R.N. and H.C.M.) independently extracted data from selected RCTs. Baseline data included: (1) maternal age; (2) gestational age; (3) TSH levels; thyroid peroxidase antibody (TPOAb) status, fertility treatment, and recurrent miscarriage. Individual patient-level data was not requested.
Our study was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement [13] and recommendations from Cochrane Collaboration Handbook for Systematic Reviews of Interventions [14]. We prospectively registered our research protocol in the International Prospective Register of Systematic Reviews (PROSPERO) on February 8, 2023 (ID CRD42023395160).
Data analysis
Outcomes evaluated were (1) pregnancy loss, defined as a composite of miscarriage and stillbirth; (2) live birth; and (3) preterm birth before 37 weeks. We also conducted subgroup analyses for live birth based on TPOAb status (positive or negative), TSH levels (2.5–4 or 4–10 mU/L), fertility treatment (with or without fertility treatment), and recurrent miscarriage (with or without recurrent miscarriage).
Quality assessment
We evaluated the risk of bias using version 2 of the Cochrane Risk of Bias Assessment Tool (RoB-2) for RCTs, where each study scored as high, moderate, or low risk of bias. The assessment was performed and documented by two independent authors (L.F.R and M.V.BM.). Disagreements were resolved through consensus after discussing reasons for discrepancy. Publication bias was assessed through the generation of funnel plots.
Statistical analysis
We considered p values of less than 0.05 to be statistically significant and computed risk ratios (RR) using the Inverse Variance test for dichotomous outcomes with 95% confidence intervals (CI) as a measure of effect size. To assess heterogeneity, Cochran’s Q test and I2 statistics were utilized. We classified I2 values of <25%, 25–75%, and >75% as representing low, moderate, and high heterogeneity, respectively. To account for potential disparities in both clinical and methodological aspects across studies, we applied random effects models, and performed subgroup analyses to investigate heterogeneity between study-specific estimates. Our meta-analysis was conducted using the meta package for RStudio version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study selection and characteristics
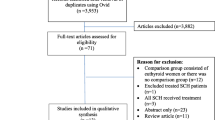
The initial search yielded 479 results. After removing duplicate studies, 371 records were identified through database searching and their summaries were screened for eligibility. Of these, 17 remained and were fully reviewed based on the predefined eligibility criteria (Fig. 1). A total of 11 RCTs were included comprising 2,749 patients, of whom 1,439 (52.3%) were in the levothyroxine group. Table 1 summarizes individual studies characteristics. Although five studies allocated participants before pregnancy [15,16,17,18,19], the other trials enrolled patients up to the second trimester. Moreover, only four RCTs encompassed pregnant women with TPOAb positivity [17,18,19,20]. The maternal age ranged from 26.9 to 36.1 years.
Pooled analyses
In a pooled analysis of 6 trials, levothyroxine therapy was associated with a significant 31% reduction of the risk of pregnancy loss compared to no therapy (RR 0.69; 95% CI 0.52–0.91; p < 0.01; 6 studies; Fig. 2). Out of the 11 studies, 8 provided information on the incidence of live birth, which showed no significant association between levothyroxine and live birth (RR 1.01; 95% CI 0.99–1.03; p = 0.29; 8 studies; Fig. 3). Furthermore, when compared with no treatment, levothyroxine was not associated with preterm birth before 37 weeks (RR 0.71; 95% CI 0.49–1.03; p = 0.07; 6 studies; Figure S1). There was no significant interaction in live birth across all subgroup analyses (p > 0.05) and no subgroup demonstrated an increase in this outcome compared with no treatment (Figs. 4 and 5).
Quality assessment
Individual RCT appraisal is shown in Figure S2. No study was considered to have a high risk of bias. Although the limited number of included studies, there was no evidence of publication bias based on the funnel plots (Figures S3 to S5).
Discussion
In this systematic review and updated meta-analysis of 11 RCTs and 2,749 patients with SCH, we compared levothyroxine with placebo or no treatment, evaluating pregnancy loss, live birth, and preterm birth. Our main findings were: (1) a significantly reduced risk of pregnancy loss with the use of levothyroxine; (2) no significant association between levothyroxine and live birth or preterm birth; and (3) no significant interaction between all subgroups analyzed.
Pregnant women diagnosed with SCH are at a higher risk of experiencing adverse outcomes, such as preterm delivery, hypertensive disorders of pregnancy, and preeclampsia. More specifically, women with untreated SCH in early pregnancy show a 1.9-fold risk of miscarriage compared to euthyroid subjects [7]. The condition also has important implications for neonatal outcomes, such as an elevated risk of intellectual disability [21]. However, there is no current consensus on the effectiveness of thyroid hormone replacement therapy in pregnant women with SCH to prevent such adverse outcomes [22]. Observational and randomized data present conflicting conclusions, and as a result, various scientific bodies, including the ATA and the European Thyroid Association report weak or insufficient evidence on the effectiveness of thyroxine treatment in pregnancy and neonatal outcomes [9, 23].
To the best of our knowledge, this is the first meta-analysis to explore the efficacy of levothyroxine in different subgroups of clinical interest. Our finding of pregnancy loss was consistent with prior meta-analyses that were performed adopting the old 2011 ATA criteria, such as those of Rao et al., and Nazarpour et al., which also found a reduced risk of pregnancy loss with the use of levothyroxine [24, 25]. However, more recent meta-analyses based on the 2017 ATA criteria, such as the one performed by Jiao and colleagues [11], found no differences in maternal and neonatal outcomes between groups when restricted to RCT data. This can be attributed to the fact that studies in their analysis did not have enough statistical power to reach firm conclusions, which was confirmed by their trial sequential analysis. In addition to having included studies with both 2011 and 2017 ATA criteria for a higher power in our analyses, we have also included recent RCTs published since prior reviews were conducted, resulting in a larger pooled population and thus, more accurate conclusions on the management of SCH.
This meta-analysis provides an up-to-date synthesis of published RCTs that were not previously included in other systematic reviews and meta-analyses, and the inclusion of only RCTs minimizes the effect of confounding factors. In addition, different subgroup analyses provide data that are applicable in a range of clinical settings. Nonetheless, our findings must be interpreted in the context of our study’s limitations. First, it is important to note that some analyzes were considered with significant heterogeneity. However, this finding was expected in view of the variation in starting doses of levothyroxine therapy and mean gestational age. To minimize and interpret such heterogeneities, we conducted subgroup analyses for each ATA criteria, TPOAb status, fertility treatment and recurrent miscarriage. We cannot, however, eliminate the impact of other clinical factors that may have resulted in the observed discrepancy between studies. Furthermore, individual participant-level data was not requested, limiting our ability to further delineate the effect of variables such as race and geographical location which have been suggested to greatly impact the variability of TSH levels [26].
Conclusion
Although our meta-analysis has shown that treatment with levothyroxine led to a significant reduction in the risk of pregnancy loss, this finding contrasts with the results of live birth and preterm birth, in addition to substantial heterogeneity in certain subgroups. Additional analyses are warranted for individuals exhibiting thyroid stimulating hormone levels exceeding four milliunits per liter, particularly when associated with recurrent miscarriage or infertility.
References
Cooper DS (2001) Subclinical hypothyroidism. N Engl J Med 345(4):260–265. https://doi.org/10.1056/NEJM200107263450406
Shan ZY, Chen YY, Teng WP et al (2009) A study for maternal thyroid hormone deficiency during the first half of pregnancy in China. Eur J Clin Invest 39(1):37–42. https://doi.org/10.1111/j.1365-2362.2008.02055.x
Dhanwal D, Prasad S, Agarwal A, Dixit V, Banerjee A (2013) High prevalence of subclinical hypothyroidism during first trimester of pregnancy in North India. Indian J Endocrinol Metab 17(2):281. https://doi.org/10.4103/2230-8210.109712
Karcaaltincaba D, Ozek MA, Ocal N, Calis P, Inan MA, Bayram M (2020) Prevalences of subclinical and overt hypothyroidism with universal screening in early pregnancy. Arch Gynecol Obstet 301(3):681–686. https://doi.org/10.1007/s00404-020-05462-0
Sohail R, Yasmin H, Tasneem N, et al (2021) The prevalence of subclinical hypothyroidism during early pregnancy in Pakistan: a cross-sectional study. Cureus. Published online December 10, 2021. https://doi.org/10.7759/cureus.20316
Casey BM, Dashe JS, Wells CE et al (2005) Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 105(2):239–245. https://doi.org/10.1097/01.AOG.0000152345.99421.22
Zhang Y, Wang H, Pan X, Teng W, Shan Z (2017) Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: a systematic review and meta-analysis. PLoS ONE 12(4):e0175708. https://doi.org/10.1371/journal.pone.0175708
Cakmak BD, Turker UA, Temur M, Ustunyurt E (2019) Pregnancy outcomes of antibody negative and untreated subclinical hypothyroidism. J Obstet Gynaecol Res 45(4):810–816. https://doi.org/10.1111/jog.13925
Alexander EK, Pearce EN, Brent GA et al (2017) 2017 Guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389. https://doi.org/10.1089/thy.2016.0457
Stagnaro-Green A, Abalovich M, Alexander E et al (2011) Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21(10):1081–1125. https://doi.org/10.1089/thy.2011.0087
Jiao XF, Zhang M, Chen J et al (2022) The impact of levothyroxine therapy on the pregnancy, neonatal and childhood outcomes of subclinical hypothyroidism during pregnancy: an updated systematic review, meta-analysis and trial sequential analysis. Front Endocrinol 13:964084. https://doi.org/10.3389/fendo.2022.964084
Ding Z, Liu Y, Maraka S et al (2021) Pregnancy and neonatal outcomes with levothyroxine treatment in women with subclinical hypothyroidism based on new diagnostic criteria: a systematic review and meta-analysis. Front Endocrinol 12:797423. https://doi.org/10.3389/fendo.2021.797423
Page MJ, McKenzie JE, Bossuyt PM et al (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112. https://doi.org/10.1016/j.jclinepi.2021.02.003
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2019) Cochrane handbook for systematic reviews of interventions. Published online September 23, 2019
Kim CH, Ahn JW, Kang SP, Kim SH, Chae HD, Kang BM (2011) Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 95(5):1650–1654. https://doi.org/10.1016/j.fertnstert.2010.12.004
Rahman AHA, Abbassy HA, Abbassy AAE (2010) Improved in vitro fertilization outcomes after treatment of subclinical hypothyroidism in infertile women. Endocr Pract 16(5):792–797. https://doi.org/10.4158/EP09365.OR
Van Dijk MM, Vissenberg R, Fliers E et al (2022) Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 10(5):322–329. https://doi.org/10.1016/S2213-8587(22)00045-6
Wang H, Gao H, Chi H et al (2017) Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA 318(22):2190. https://doi.org/10.1001/jama.2017.18249
Dhillon-Smith RK, Middleton LJ, Sunner KK et al (2019) Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med 380(14):1316–1325. https://doi.org/10.1056/NEJMoa1812537
Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Alavi Majd H, Azizi F (2017) Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 176(2):253–265. https://doi.org/10.1530/EJE-16-0548
Thompson W, Russell G, Baragwanath G, Matthews J, Vaidya B, Thompson-Coon J (2018) Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. Clin Endocrinol 88(4):575–584. https://doi.org/10.1111/cen.13550
Urgatz B, Razvi S (2023) Subclinical hypothyroidism, outcomes and management guidelines: a narrative review and update of recent literature. Curr Med Res Opin 39(3):351–365. https://doi.org/10.1080/03007995.2023.2165811
Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B (2014) 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 3(2):76–94. https://doi.org/10.1159/000362597
Nazarpour S, Ramezani Tehrani F, Amiri M, Bidhendi Yarandi R, Azizi F (2019) Levothyroxine treatment and pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. Arch Gynecol Obstet 300(4):805–819. https://doi.org/10.1007/s00404-019-05245-2
Rao M, Zeng Z, Zhou F et al (2019) Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: a systematic review and meta-analysis. Hum Reprod Update 25(3):344–361. https://doi.org/10.1093/humupd/dmz003
Bliddal S, Feldt-Rasmussen U, Boas M et al (2014) Gestational age-specific reference ranges from different laboratories misclassify pregnant women’s thyroid status: comparison of two longitudinal prospective cohort studies. Eur J Endocrinol 170(2):329–339. https://doi.org/10.1530/EJE-13-0672
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Henrique Provinciatto, Marcus Vinicius Barbosa Moreira and Gabriel Rezende Neves. The first draft of the manuscript was written by Henrique Costa Mitsui, Julio Min Fei Zhang and Edward Araujo Júnior and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Provinciatto, H., Moreira, M.V.B., Neves, G.R. et al. Levothyroxine for subclinical hypothyroidism during pregnancy: an updated systematic review and meta-analysis of randomized controlled trials. Arch Gynecol Obstet 309, 2387–2393 (2024). https://doi.org/10.1007/s00404-024-07512-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-024-07512-3