Abstract
Iron deficiency occurs frequently in pregnancy and can be diagnosed by serum ferritin-level measurement (threshold value < 30 μg/L). Screening for iron-deficiency anemia is recommended in every pregnant women, and should be done by serum ferritin-level screening in the first trimester and regular hemoglobin checks at least once per trimester. In the case of iron deficiency with or without anaemia in pregnancy, oral iron therapy should be given as first-line treatment. In the case of severe iron-deficiency anemia, intolerance of oral iron, lack of response to oral iron, or in the case of a clinical need for rapid and efficient treatment of anaemia (e.g., advanced pregnancy), intravenous iron therapy should be administered. In the postpartum period, oral iron therapy should be administered for mild iron-deficiency anemia (haemorrhagic anemia), and intravenous iron therapy for moderately severe-to-severe anemia (Hb < 95 g/L). If there is an indication for intravenous iron therapy in pregnancy or postpartum, iron-containing drugs which have been studied in well-controlled clinical trials in pregnancy and postpartum such as ferric carboxymaltose must be preferred for safety reasons. While anaphylactic reactions are extremely are with non-dextrane products, close surveillance during administration is recommended for all intravenous iron products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaemia is one of the most common problems in obstetrics. In Switzerland, up to 32% of all pregnant women suffer from iron deficiency and up to 7% from iron-deficiency anaemia. Up to 1/3 of all women suffer from postpartum anaemia. It is well known that, depending on its severity, anaemia constitutes an important risk factor in both maternal and foetal morbidity and mortality [1,2,3]. If the mother suffers from iron-deficiency anaemia, risks to the foetus include a higher rate of premature birth, intrauterine growth retardation, unfavourable impact on placental development, and reduced neonatal iron stores. Maternal risks include increased risk of infection, depleted blood reserves during delivery and thus an increased risk of allogeneic blood transfusion in the case of significant blood loss, cardiovascular stress, anaemia symptoms (fatigue, reduced physical and mental capacities, headaches, orthostatic dizziness, exhaustion, etc.), prolonged hospitalisations, decreased milk production in the puerperium, increased risk of postnatal depression, depleted maternal iron stores postpartum and subsequently. For these reasons, the efficient treatment of anaemia following its diagnosis has a positive impact on maternal as well as foetal outcomes. One main focus is on reducing or at best avoiding the need for an allogeneic blood transfusion, as a result of adequate anaemia treatment prior to delivery [4,5,6,7]
Anaemia in pregnancy
Diagnosis
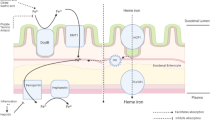
According to the WHO guidelines, the lower threshold value for haemoglobin in pregnancy is defined as Hb < 110 g/L. As Hb levels drop temporarily by 5 g/L in the second trimester, the CDC (1998) defines the lower threshold value for anaemia as < 105 g/L in the second trimester. Anaemia requires diagnostic clarification and treatment because of its association with an increased risk of maternal complications (severe peripartum anaemia requiring transfusion) and infant complications (intrauterine growth retardation, premature birth, postpartum iron deficiency in the infant and toddler with ensuing developmental problems). For diagnostic clarification, the first tests to be performed should be a red blood count and a serum ferritin assay. As a rule, determining the serum ferritin level is sufficient for the diagnosis of iron-deficiency anaemia. A value of < 30 µg/L is evidence of depleted iron stores and iron-deficiency anaemia. For normal and/or elevated serum ferritin levels, other possible causes must be investigated (e.g. haemoglobinopathies such as β-thalassaemia, sickle cell anaemia, anaemia of infection, haemorrhagic anaemia, etc.). Determining the serum ferritin level in addition to the haemoglobin level in all pregnant women at the beginning of pregnancy is a good strategy [8,9,10]. If ferritin is < 30 µg/L, there is a 90% probability that iron stores are depleted even if there is no indication as yet of anaemia. In these cases, iron therapy during pregnancy is indicated even if there is no indication (as yet) of anaemia. The reason for this is that iron requirements increase dramatically in pregnancy to cover additional maternal requirements (expansion of erythrocyte volume) and foetal requirements (building of skeleton, CNS, and foetal erythrocyte mass). Caution: as part of an inflammatory response, serum ferritin can be “false normal” to “false high”, as it reacts in the same way as an acute-phase protein. For this reason, the recommendation is to determine the CRP level at the same time as ferritin levels. Genetic haemoglobinopathies, which are more likely to occur in certain ethnic groups, represent a further significant cause of anaemia. In the following cases, the advice is to perform a haemoglobin electrophoresis or a haemoglobin chromatography (HPLC, high performance liquid chromatography) to identify β-thalassaemia or another haemoglobinopathy as the cause of anaemia: (a) positive family case history of the pregnant woman or her partner, (b) anaemia without iron deficiency (ferritin level normal) (c) an MCV (erythrocyte mean corpuscular volume) level of < 70 fL or an MCH (mean corpuscular haemoglobin) level of < 27 pg (caution: Hb electrophoresis can be normal for α-thalassaemia!) and (d) depending on ethnicity (caution: blood count for sickle cell anaemia without pathological findings). In the case of a proven—usually heterozygous—haemoglobinopathy, the partner must also be examined and the option of invasive prenatal diagnostics offered, if there is a relevant risk to the foetus [11]. If the anaemia is unexplained (especially with elevated or high–normal MCV/MCH levels), serum holotranscobalamin (vitamin B12) should be determined given that vitamin B12 deficiency is not uncommon (in particular with a vegetarian or vegan diet or hyperemesis gravidarum) and, in the case of deficiency, vitamin B12 should be substituted. The less common folic acid deficiency anaemia is associated with macrocytic megaloblastic anaemia.
Treatment of iron-deficiency anaemia in pregnancy
The choice of treatment depends on the cause of the anaemia, i.e. generally iron deficiency. Oral iron products or intravenous iron products can be used for iron therapy. Various studies have shown that, once indicated, intravenous iron therapy is superior to oral iron therapy in terms of speed and absolute extent of haemoglobin increase [12]. In addition, with oral iron therapy, clinically relevant gastrointestinal side effects (gastric intolerance, constipation) occur at a frequency of 20%, which can be avoided with intravenous iron therapy (LoE Ib). Several studies have shown the tolerance and safety of certain intravenous iron products in pregnancy (LoE Ib). Hypersensitivity reactions (skin exanthema, bronchoconstriction, possible drop in blood pressure) occur extremely rarely with the new non-dextran iron products [13,14,15,16,17]
The primary treatment for mild cases of iron-deficiency anaemia and iron deficiency without anaemia in pregnancy is peroral iron therapy (iron II salts or iron III polymaltose) at doses of 160–200 mg/day (ideally on an empty stomach, fractionated). The same applies to iron deficiency and depleted iron stores (ferritin < 30 µg/L) without anaemia at the beginning of pregnancy, because of the additional requirement for iron in the course of the pregnancy. Iron substitution with an iron dose below 100 mg/day, as is contained in certain multivitamin products (for example Elevit® with 80 mg iron), is inadequate. After 2–4 weeks, checks should be performed to see if treatment has been successful (LoE IIa)
In the following clinical situations intravenous iron therapy is indicated in pregnancy from the second trimester onwards:
-
Lack of response to oral iron (Hb levels rising by less than 10 g/L within 14 days).
-
Intolerance of oral iron products (gastrointestinal side effects) or lack of compliance.
-
Severe or advanced anaemia (Hb < 90 g/L).
-
Need for rapid and efficient anaemia treatment (advanced gestational age, placenta praevia, Jehovah’s Witness, etc.).
Selection of intravenous iron product
Ferric carboxymaltose (Ferinject®): Based on available study data, Ferinject is the first-choice product when intravenous iron therapy is indicated in pregnancy. Since the last update of the 2009 expert letter, several randomised studies, some of them large, have shown that Ferinject is a safe and effective intravenous iron product in pregnancy. There are now six published studies available on the use of Ferinject in pregnancy in a total of 634 pregnant women with iron-deficiency anaemia [13, 14, 16, 18, 19]. In all the studies, Ferinject was superior to the comparative products (oral iron, iron saccharate complex, and iron dextran) in terms of efficacy, and displayed a very low rate of undesirable side effects. No serious intolerance reactions (anaphylactic shock) were described in the studies after administration of ferric carboxymaltose. The first large randomised controlled multicentre study with Ferinject in pregnancy was recently published [13]. The study shows that women treated with Ferinject benefited not only from a more rapid and more efficient Hb increase compared to the oral iron group, but also from a clearly improved quality of life (LoE Ib). No undesirable effects were shown in the newborns of women treated with Ferinject. Ferinject should be administered at weight-adapted doses of up to 1000 mg in a rapid infusion over a short period of time (15–30 min per infusion). A controlled study comparing ferric carboxymaltose (Ferinject) and iron saccharate (Venofer) showed the superiority of Ferinject in terms of the intravenous dose (1000 mg per rapid infusion) with equivalent levels of tolerance [14]. This makes it possible to avoid costly repeat infusions of small intravenous iron quantities. Ferinject is approved for administration in the second and third trimester of pregnancy. An ex vivo placental perfusion study showed that ferric carboxymaltose does not cross the placental barrier [Malek, 2009]. Ferric carboxymaltose is generally administered as a rapid infusion over 15–30 min at a dose of 1000 mg (maximum 20 mg per kg body weight). If higher doses (> 1000 mg) are required, they must be fractionated and administered at intervals of at least 7 days. Please see the drug compendium for further details on the use of Ferinject.
As an alternative to ferric carboxymaltose or if Ferinject® is not available, other non-dextran intravenous iron products, such as iron III saccharate (Venofer®), may be used as a second choice.
Ferritin levels should not be determined in the first 3–4 weeks after intravenous iron therapy as levels increase rapidly and significantly after intravenous administration and then decrease slowly in the medium term.
Precautionary measures for intravenous iron therapy
The precautionary measures recommended by Swissmedic for intravenous iron therapy should be adhered to in general and in pregnancy in particular. Please see the following two links in this context:
-
https://www.swissmedic.ch/marktueberwachung/00135/00157/01684/index.html?lang=de&download=NHzLpZeg7t,lnp6I0NTU042l2Z6ln1acy4Zn4Z2qZpnO2Yuq2Z6gpJCDdn92g2ym162epYbg2c_JjKbNoKSn6A–
-
https://www.swissmedic.ch/aktuell/00673/00688/01489/index.html?lang=de
Extravasation should be avoided due to the risk of long-term skin discolouration. This is why careful and frequent observation of the infusion site is recommended during the iron infusion. In the case of extravasation, discontinue the infusion immediately (do not rinse with NaCl!) and issue a pharmacovigilance report.
Patient blood management: avoiding blood transfusion
Various studies have shown that avoiding perioperative blood transfusion improves the morbidity and mortality associated with various operations (LoE Ia). In addition to avoiding unnecessary transfusion, an important strategy here consists of preoperative optimisation of haemoglobin and iron reserves in cases of elective surgery. Although there are currently very few studies on this topic in obstetrics, the situation for a planned caesarean section differs only to a limited extent from elective orthopaedic surgery, for example. In planned caesarean sections with expected high blood loss (placenta praevia, placenta increta, large myoma, etc.), high-dose intravenous iron therapy should be considered towards the end of pregnancy (depending on ferritin levels) to achieve the highest possible baseline haemoglobin level and to avoid perioperative blood transfusion [20, 21]
Postpartum anaemia
Diagnosis
An Hb level of < 120 g/L is seen as postpartum anaemia and a level of < 100 g/L as clinically significant postpartum anaemia. This is a combination of haemorrhagic anaemia and in some instances pre-existing iron-deficiency anaemia.
The decision to check Hb levels during the puerperium should be made subject to blood loss and the clinical state of the puerpera (symptoms of anaemia). The prepartum Hb level is also of relevance.
The nadir of the postpartum Hb level is reached approximately 48 h after the primary plasma volume distribution. The additional determination of the ferritin level after birth does not make sense because, for the first few weeks after delivery, serum ferritin levels may be “false normal” or “false high” (see above: ferritin = acute-phase protein). The iron stores of a puerpera can be assessed before delivery or from about 6 weeks after. There is no point in determining ferritin levels in cases of prepartum and postpartum anaemia because depleted iron stores can safely be assumed. Parenteral iron treatment without previous ferritin assessment may be dangerous in cases of haemochromatosis (heterozygous frequency: 1:10).
Treatment of postpartum anaemia
Treatment options for postpartum iron-deficiency anaemia comprise oral iron administration, intravenous iron therapy, erythropoietin therapy, and blood transfusion. These treatment options are discussed in the following.
Intravenous iron therapy is superior to oral iron therapy due to the more rapid Hb increase, higher absolute Hb level, improvement in fatigue score, and lower rate of gastrointestinal side effects. Various randomised studies have shown an advantage for intravenous iron therapy compared to oral iron [17, 19, 22] (LoE Ia). One study even showed that the introduction of parenteral iron led to a reduction in allogeneic blood transfusion within the study collective. The theoretical, extremely low risk of a hypersensitivity reaction must be considered as a potential disadvantage and taken into account when selecting the treatment method.
Treatment generally depends on the severity of the anaemia and the puerpera’s state of health
-
For mild anaemia (Hb 95–120 g/L): peroral administration of about 80–200 mg iron (iron II salts or iron III polymaltose).
-
In the case of poor (gastrointestinal) tolerance of the peroral iron therapy: switch to intravenous iron administration.
-
For moderately severe (Hb 85–95 g/L) or severe (Hb < 85 g/L) anaemia: intravenous iron administration as first choice.
Selection of intravenous iron product: If postpartum intravenous iron therapy is indicated, the recommended first-choice product is ferric carboxymaltose (Ferinject®), which has undergone intensive investigations. It has already been tested in several randomised multicentre studies in comparison with oral iron substitution for the treatment of postpartum anaemia, and demonstrated an outstanding safety profile combined with great efficacy. In three of the four studies, the intravenous administration of ferric carboxymaltose for the treatment of postpartum anaemia showed superiority in efficacy (Hb increase, maximum Hb value) to oral iron therapy, while one study showed ferric carboxymaltose to be equal to oral iron therapy over 12 weeks (LoE Ib). Compared to other iron products, the non-dextran product has the advantage of causing only extremely rare hypersensitivity reactions. In comparison with iron saccharate (Venofer), which is also well-tolerated, there is also the advantage of a significantly higher maximum dose (up to 1000 mg per rapid infusion for Ferinject versus up to 200 mg per rapid infusion for Venofer). A recently published retrospective comparative study showed greater efficacy with the same rate of side effects (Pfenninger et al., J Perinat Med 2012). Practical benefits, patient comfort and the reduction in costs associated with a single administration support the advantage of ferric carboxymaltose over iron III saccharate (Venofer). This is why ferric carboxymaltose (Ferinject) is the first-choice product for the treatment of postpartum iron-deficiency anaemia. It can be administered as a rapid infusion at a dose of up to 1000 mg, which can be repeated at weekly intervals (depending on the Hb level).
As an alternative to ferric carboxymaltose or if Ferinject® is not available, other non-dextran intravenous iron products, such as iron III saccharate (Venofer®), may be used as a second choice.
In cases of severe anaemia (< 80 g/L), the administration of recombinant erythropoietin (rhEPO) in addition to parenteral ferric carboxymaltose may be considered. According to the Cochrane database, the administration of rhEPO can support the treatment of anaemia, but only in conjunction with parenteral iron to avoid an ineffective erythropoiesis. However, there is only extremely limited evidence for additional efficacy of rhEPO in combination with intravenous iron therapy versus intravenous iron therapy alone. (LoE IIa) RhEPO should, therefore, only be given if at all in cases of severe anaemia combined with additional factors (pronounced clinical symptoms, rejection of donor blood, etc.). A sample dose is, e.g. 150 IU/kg body weight once a day s.c., a total of four doses of epoietin alpha (Eprex®), in addition to the parenteral treatment with ferric carboxymaltose. Consideration must also be given to the fact that the administration of rhEPO is an off-label use with considerable associated costs.
The critical Hb value below which allogeneic blood transfusion should be performed is approximately 60–65 g/L, but depends on clinical symptoms. A decision on allogeneic blood transfusion should always be made on an individual basis giving full consideration to the patient’s wishes. There is no general threshold value (e.g. Hb 60 g/L = blood transfusion), but consideration must be given to inapparent complications, such as indications of silent myocardial ischaemia.
Summary
-
Iron deficiency occurs frequently in pregnancy and can be diagnosed in the first trimester by means of serum ferritin screening (threshold value < 30 µg/L). Regular Hb checks at least once per trimester are generally recommended (GoR B)
-
In the case of iron deficiency with or without anaemia in pregnancy, oral iron therapy should be given as first-line treatment. In the case of severe iron-deficiency anaemia, intolerance of oral iron, lack of response to oral iron, or in the case of a clinical need for rapid and efficient treatment of anaemia, intravenous iron therapy should be administered (GoR A).
-
In the postpartum period, oral iron therapy should be administered for mild iron-deficiency anaemia (haemorrhagic anaemia), and intravenous iron therapy for moderately severe to severe anaemia (Hb < 95 g/L) (GoR A).
-
If there is an indication for intravenous iron therapy in pregnancy or postpartum, ferric carboxymaltose is the first-choice product based on existing studies and our own experience. Particular care is recommended for all intravenous iron products in accordance with the Swissmedic information documents (GoR C).
Date: 06.01.2017

(Source: RCOG guidelines no. 44, 2006)
References
WHO Global Database. Worldwide prevalence of anemia 1993–2005; 2008
Bencaiova G, Breymann C (2014) Mild anemia and pregnancy outcome in a Swiss collective. J Pregnancy 2014:307535
Hercberg S, Preziosi P, Galan P (2001) Iron deficiency in Europe. Public Health Nutr 4:537–545
Allen LH (1997) Pregnancy and iron deficiency: unresolved issues. Nutr Rev 55(4):91–101
Breymann C (2015) Anemia and iron deficiency anemia in gynecology and obstetrics, vol 1. Bremen
Murray-Kolb L (2012) Maternal mortality, child mortality, perinatal mortality, child cognition, and estimates of prevalence of anemia due to iron deficiency. CHERG
Rondo P, Tomkins A (1999) Maternal iron status and intrauterine growth retardation. Trans RS Trop Med 93:423–426
Gibson RS (1990) Assessment of iron status. Principles of nutritional assessment. Oxford University Press, Oxford, pp 349–376
Milman N, Agger A, Nielson O (1991) Iron supplementation during pregnancy. Dan Med Bull 38:471–476
Milman N, Graudal N, Agger A (1995) Iron status markers during pregnancy: no relationship between levels at the beginning of the second trimester, prior delivery and post partum. J Int Med 237:261–267
Krafft A, Breymann C (2004) Haemoglobinopathy in pregnancy: diagnosis and treatment. Curr Med Chem 11(21):2903–2909
Breymann C, Bian XM, Blanco-Capito LR, Chong C, Mahmud G, Rehman R (2011) Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J Perinat Med 39(2):113–121
Breymann C, N NM, Mezzacasa A, Bernard R, Dudenhausen J, investigators F-A (2016) Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinat Med (epub ahead of print)
Christoph P, Schuller C, Studer H, Irion O, Tejada BD, Surbek D (2012) Intravenous iron treatment in pregnancy: comparison of high-dose ferric carboxymaltose vs. iron sucrose. J Perinat Med 13(40):469–474
Froessler B, Cocchiaro C, Saadat-Gilani K, Hodyl N, Dekker G (2013) Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: a randomized trial. J Matern Fetal Neonatal Med 26(7):654–659
Froessler B, Collingwood J, Hodyl NA, Dekker G (2014) Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Pregnancy Childbirth 14:115
Gupta A, Manaktala U, Rathore AM (2014) A randomised controlled trial to compare intravenous iron sucrose and oral iron in treatment of iron deficiency anemia in pregnancy. Indian J Hematol Blood Transfus 30(2):120–125
Breymann C, Gliga F, Bejenariu C, Strizhova N (2008) Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. Int J Gynaecol Obstet 101(1):67–73
Rathod S, Samal SK, Mahapatra PC, Samal S (2015) Ferric carboxymaltose: a revolution in the treatment of postpartum anemia in Indian women. Int J Appl Basic Med Res 5(1):25–30
Gravier A (1999) Avoiding postpartum transfusions: the utility of intravenous iron supplementation (translation). J Gynecol Obstet Biol Reprod 28:77–78
Muñoz M, Breymann C, García-Erce J, Gómez-Ramírez S, Comin J, Bisbe E (2008) Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox San 94(3):172–183
Gupta A, Rathore AM, Manaktala U, Gupta A, Gupta S (2015) Role of intravenous iron sucrose in correction of anemia in antenatal women with advanced pregnancy. Indian J Hematol Blood Transfus 31(2):251–254
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Swiss Society of Gynecology and Obstetrics (SSGO).
Conflict of interest
C. Breymann has received speaker and workshop honorarium, and clinical study support from Vifor Inc. C. Honegger declares that he has no conflict of interest. I. Hösli has received speaker honorarium from Vifor Inc. D. Surbek has received speaker honorarium and an unrestricted grant for a investigator-initiated study from Vifor Inc.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
This expert recommendation has been reviewed by the members of the Quality assurance committee of the SSGO. The Quality Assurance Committee of gynécologie suisse/SGGO (Swiss Society of Obstetrics and Gynaecology) compiles guidelines and expert letters with utmost care. However, the Quality Assurance Committee of gynécologie suisse/SGGG accepts no responsibility for the correctness and completeness of the content. The manufacturer’s instructions must be observed at all times, in particular the dosage instructions. To the Committee’s best knowledge, guidelines and expert letters correspond to the latest scientific insights at the time of publication. Users must take intervening changes into account.
Additional information
Expert panel recommendation from the Quality Assurance Committee, Swiss Society of Gynecology and Obstetrics SSGO.
Rights and permissions
About this article
Cite this article
Breymann, C., Honegger, C., Hösli, I. et al. Diagnosis and treatment of iron-deficiency anaemia in pregnancy and postpartum. Arch Gynecol Obstet 296, 1229–1234 (2017). https://doi.org/10.1007/s00404-017-4526-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4526-2