Abstract
Background and objective
Primary dysmenorrhea starts simultaneously with menstruation or before it and usually continues for 48–72 h. As a prevalence disorder, it affects about 80–97% of women in the reproductive age. The conventional treatment modalities of primary dysmenorrhea are associated with complications and side effects. In addition, there is a lack of knowledge of the effect of honey on the treatment of primary dysmenorrhea. The objective of this study is to investigate the effect of honey on the severity of pain in women with dysmenorrhea.
Methods
A randomized crossover clinical trial was conducted on 56 female students. Subjects were randomly assigned to two groups. Groups I and II received honey and mefenamic acid in the ‘first treatment period’, respectively. In the ‘second treatment period’, the intervention methods were reversed between the groups. Samples recorded the severity of pain during the first 3 days of menstruation.
Results
There were no significant differences in the most severe level of pain in the first and second months of the first treatment period, and the first and second months of the second treatment period between the groups.
Conclusions
Honey and the mefenamic acid capsules led to the same amount of pain relief in women with primary dysmenorrhea. Honey is suggested to be used for pain relief due to its lower side effects and pharmacological complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary dysmenorrhea as a prevalent disorder affects 80–97% of women. In 15.4% of the women, primary dysmenorrhea can lead to the limitation of activity, impairment of efficiency, and absence from the workplace [1]. Dysmenorrhea is a disorder with personal and social health consequences leading to 600 million-work hour’s loss and the subsequent economic loss about 2 billion dollars per year in the USA [2].
Primary dysmenorrhea has no clear cause and it is described as the occurrence of painful menstruation according to women’s health record file and clinical examination. Secondary dysmenorrhea has often caused by endometriosis, adenomyosis, uterine fibroids, and infection [2, 3]. In addition, a reduction of progesterone in the final stages of luteal phase leads to tearing the lysosome apart, and consequently, the release of phospholipase A2 from endometrium has been proposed as a possible cause of primary dysmenorrhea. It is noted that phospholipase A2 triggers the augmentation of prostaglandin that in return results in the contraction of uterine muscles and arteries, which finally gives rise to uterine ischemia and pain [4].
Various pharmaceutical, surgical, and complimentary medical methods are used for the treatment of primary dysmenorrhea [1, 3, 5, 6]. The main pharmaceutical modalities are oral contraceptive pills, nonsteroidal anti-inflammatory drugs, calcium channel blockers, progesterone, and so on [4]. Beside their beneficial effects, the respective chemical medicines may cause side effects including heartburn, blurred vision, dizziness, headache, constipation, diarrhea, fatigue, dysuria, drowsiness, anorexia, nausea, skin acne, vomiting, and gastrointestinal bleeding [2]. Therefore, the selection of a medicine with fewer side effects is of the utmost importance to healthcare professionals.
Nowadays, a tendency has grown to use herbs, supplementary and alternative medications for the treatment of primary dysmenorrhea. Honey as a food with high nutritional values contains 20 types of sugar, 8 types of vitamins, 11 types of minerals, 16 types of amino acids, several types of enzymes, and many other unknown substances [7]. Islamic texts have recommended the intake of honey as a healing medicine; for instance, the Holy Quran in Surah Nahl, Verse 69 says: “Some nectar extrudes out of honey bee, which is healing for the people” [8]. Anti-prostaglandin properties of honey have been demonstrated in different studies [9, 10]. Honey has been recognized as a well known and widely accepted as a nutritional source in the Iranian society.
The prevalence of primary dysmenorrhea and the associated complications of the current pharmaceutical products used for the treatment of this disease have increased the public attention to supplementary and herbal medications. In addition, there is a lack of knowledge about the effect of honey on the treatment of primary dysmenorrhea. The novelty of this topic and insufficient studies on the effect of honey on primary dysmenorrhea motivated the researchers to conduct this study with the aim of investigating the effect of honey on the severity of pain in women with primary dysmenorrhea. The results of the present study can improve our understanding of how to promote women’s health.
Materials and methods
Design
A randomized clinical trial using a crossover design was conducted between May 2013 and March 2014. The samples were female students studying in an urban area of Iran in Arak University. The inclusion criteria were: single, having regular menstruation cycles with a 21–35 day interval, experiencing dysmenorrhea in the most of menstruation cycles, onset of pain with menstruation bleeding, and type 2 and 3 dysmenorrhea according to the verbal multidimensional criteria [12, 13]. In addition, abnormal results reported by the ultrasound of the reproductive system, abnormal uterine bleeding, the ovarian cyst disorder, history of surgery in the reproductive system, the intake of hormonal medicines, and oral contraceptive pills in the last 3 months, following weight loss diets, hydrotherapy, vegetarianism, etc., having mental and psychological tensions such as marriage, divorce, and death of parents during the last 3 months were considered exclusion criteria.
For selecting the samples, an information flyer was distributed among all students in the selected dormitories. Individuals who were interested in participating in the study contacted the researcher and then were evaluated against inclusion and exclusion criteria. Individuals who met the inclusion criteria and showed symptoms of primary dysmenorrhea were considered to receive the intervention. Individuals with symptoms of secondary dysmenorrhea were referred to a specialist for further investigation and treatment.
Samples and setting
Considering the number of samples in the analogous interventional studies [11] and also considering the probability of sample size reduction, β = 0.2 and α = 0.05, the number of samples was estimated equal to 50 persons in total and 25 persons in each group. Considering the likelihood of sample size shrinkage in both groups, 30 persons were selected for each group according to the inclusion criteria.
Ethical considerations
The study’s research proposal was approved by the research council affiliated with Arak University of Medical Sciences that provided financial support. In addition, this study was registered at the Ethics Committee of Arak University of Medical Sciences that corroborated its ethical considerations (decree number 89-94-3).
The samples were informed of the study method. They were assured of the confidentiality of their identity during the study and the possibility of withdrawal at any time without being penalized. Finally, those who agreed to participate in this study provided written informed consent.
Data collection
The samples were asked to complete the questionnaire related to demographic data and menstruation cycle. Before the interventions as the control cycle, no intervention was performed, and merely, the participants were asked to record the most severe level of pain that would experience during the first, second, and third days of the menstrual cycle using the visual analogue scale (VAS) from 0 = no pain to 10 = intolerable pain. They were asked to record the severity of their pain before taking pain killer drugs, and also after taking the drugs. The VAS is a reliable and valid tool to determine the severity of pain. The reliability of this scale has been confirmed in numerous studies [14, 15]. In addition, they were asked to register the intake amount of pain killer drugs and the severity of menstruation pain in accordance with the verbal multidimensional scoring system (VMS). The VMS grading system with a four-point scale ranges from zero to three evaluates working ability, systematic symptoms, and the necessity of taking analgesic [12, 13]. This study was resumed in case of suffering from type 2 and 3 mensuration pain severity based on the VMS. In addition, data regarding any improvement in the menstruation-related symptoms and the level of satisfaction of the respective intervention were collected after the first period of intervention.
Intervention
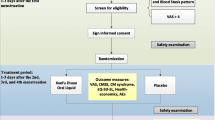
The participants were randomly assigned to two groups using the flinging coin technique; each group consisted of 30 persons. Of the participants, two persons of each group left the study due to a lack of interest to collaborate. Therefore, the study was continued with 28 people in each group (Fig. 1).
This study consisted of the first and second periods of the intervention; in the first period of intervention, one group received honey and the second group received mefenamic acid capsules. Honey group received 1.2 mg/kg honey from the 15th day to the onset of menstruation for 2 consecutive months [9, 16, 17]. Honey used in the present research was a natural honey product made of Astragalus nectar in Ashtian Region of Iran. The respective honey contained 1.5% sucrose, the fructose-to-glucose ratio of 1.2, moisture content 14%, positive diastasis, and pre-hydrolysis sugar content 67.2%. All three above-mentioned matters were prepared by a reliable honey producer in a natural process. The mefenamic acid capsules’ group received 250-mg mefenamic acid capsules manufactured by Amin Medicine Company®. They were asked to start taking medicine as soon as the first symptom of menstruation onset appeared with the following order: two capsules in the first time and then one capsule every 6 h that would be continued in the first 2 days of the first and second months. Similar to the control cycle, all samples were asked to record the severity of their pain before taking pain killer drugs, and also after taking the drugs using the VAS.
In the next menstrual cycle as the wash-out period, both groups did not receive any intervention, but recorded the required data. After the wash-out period, the ‘second treatment period’ was started, in which the intervention methods were reversed between the groups; i.e., the honey group and mefenamic acid capsules group received mefenamic acid capsules and honey in the first 2 months, respectively. The samples were provided with a telephone number so as to call the researcher in case of any further inquiry.
Data analysis
The SPSS software v. 21 for Windows (SPSS Inc., Chicago, IL, USA) was used for data analysis. The t student test and Chi-square test were applied for comparing the mean values of quantitative and qualitative variables, respectively. The Friedman and Wilcoxon tests were used to compare the level of pain severity within each group. In addition, the Mann–Whitney U test was used for inter-group comparison of the same parameter. p < 0.5 was denoted statistically significant.
Results
Demographic characteristics of the samples
The demographic and menstruation cycles’ information of the participants is presented in Table 1. The groups were similar in terms of demographic and menstruation cycles’ information characteristics. The mean age of the samples was 22.01 years (SD 1.78 years). The mean age of the samples at menarche was 13.2 years (SD 1.4 years). The mean BMI of the women was 21.2 kg/m2 (SD 3.8). The majority of the women (89.29%) had a bachelor degree. The level of sufficiency of their monthly income was more than three out four. Most of the participants (73.21%) had moderate amount of menstrual bleeding. In addition, 67.85% of the students had a family history of dysmenorrhea and 28.75% of them had dysmenorrhea in all menstrual cycles.
No statistically significant difference was found between the groups in terms of the average scores of pain in the control cycle, the first and second months of the first treatment period, wash-out period, and the first and second months of the second treatment period (Fig. 2).
The comparison of intra-group variations of pain between the honey and the mefenamic acid capsules’ groups in the first treatment period and after the wash-out period showed a statistically significant difference (Table 2).
According to Table 2, all intra-group comparisons except the comparison between the second month and the wash-out period in the mefenamic acid capsule group were statistically significant (p < 0.001). However, in the honey group, only the comparison between the first month and the control cycle and also the second month and the control cycle were statistically significant (p < 0.001).
In addition, no statistically significant difference was observed between the groups in terms of amount of bleeding, absence days from the university classes due to dysmenorrhea, a feeling of satisfaction with receiving the intervention, resumption of the deployed method in subsequent menstruations, recommendation of the method to others, and the need to other sorts of analgesics.
Discussion
This study was carried out to investigate the effect of honey on the severity of pain in women with primary dysmenorrhea. Few studies have been conducted so far to assess anti-prostaglandin effects of honey.
Our findings demonstrated that after the intervention, no statistically significant difference was found in the severity of pain in the first and second months of the intervention, and following the wash-out period in both groups. In addition, the same condition was observed after exchanging the treatments between the groups. Our results suggested that both groups equally benefitted from the intake of honey and mefenamic acid capsules. In the study by Mirbagher et al. on the comparison of sedative effects of pure honey and impure honey using a crossover design, it was shown that pure honey significantly relieved menstruation pain compared to impure honey. As a limitation, both groups only received pure or impure honey and the amounts of honey were equal for all participants. In addition, the type of honey used in that study and its elements and properties were not specified [11].
The anti-prostaglandin effects of honey have been analyzed in a few studies. Al-Waili and Boni assessed the effect of natural honey on the levels of prostaglandins E2 & F2α and urinary thromboxane B2. They indicated that the average serum concentration of thromboxane B2 decreased for 7, 34, and 35% within 3 h after the intake of honey syrup. This reduction was equal to 14, 10, and 19% in prostaglandin E2 and equal to 31 and 14% for prostaglandin F2α after 2 and 3 h of honey intake. In the 15th day, the plasma concentrations of thromboxane B2, prostaglandin E2, and prostaglandin F2α decreased for 48, 63, and 50%, respectively. They finally concluded that honey might lead to the reduction of prostaglandin levels in normal individuals [10].
Honey is a substance with high nutritional values [7] and the Islamic texts have recommended the intake of honey as a healing medicine [8]. Considering the anti-prostaglandin properties of honey and considering the role of endogenic prostaglandins as the leading cause of dysmenorrhea, it might be asserted that honey has affected the level of individuals’ menstruation pain via reducing the amount of production of prostaglandins. However, more studies are required to measure the level of prostaglandins in the blood to establish this conclusion.
In the present study, the researchers tried to present stronger results regarding the effect of honey on the level of pain in women with dysmenorrhea through the application of a relatively sufficient sample size, the deployment of standard tools for the assessment of the symptoms related to primary dysmenorrhea, a random allocation of samples into two groups, use of crossover technique, and periodical control of intervening variables along the study to ensure of the continuity of intervention trends. While it was impossible to create a placebo group for honey or use the blinding method owing to special identity of the study, the person dealing with the statistical analysis was completely blinded to the interventions applied to the groups. Volunteer sampling in this study, it will be biased towards a certain type of person as only people with a personal interest in the research topic will be volunteer. Therefore, the sample will not be truly representative of the target population or all students with primary dysmenorrheal.
Conclusions
The results of this study did not support the superiority of pain killing effects of honey in the improvement of dysmenorrhea pain comparing to mefenamic acid capsules. Nevertheless, having equal impacts to mefenamic acid capsules in terms of pain relief can be considered some evidence of its usefulness. Considering the benefits of honey and its lower side effects and pharmacological complications, it is suggested to be used as a substitute for drugs mitigating pain in women with primary dysmenorrhea.
References
Ju H, Jones M, Mishra G (2013) The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 26:mxt009
Nevatte T, O’Brien PM, Bäckström T, Brown C, Dennerstein L, Endicott J, Epperson CN, Eriksson E, Freeman EW, Halbreich U, Ismail K (2013) ISPMD consensus on the management of premenstrual disorders. Arch Women’s Mental Health 16(4):279–291
Berek JS (2011) Berek and Novak’s gynecology, 15th edn. Lippincott, New York
Ryan KJ (ed) (1999) Kistner’s gynecology and women’s health. Mosby Inc, St. Louis
Durain D (2004) Primary dysmenorrhea: assessment and management update. J Midwifery Women’s Health 49(6):520–528
Polat A, Celik H, Gurates B, Kaya D, Nalbant M, Kavak E, Hanay F (2009) Prevalence of primary dysmenorrhea in young adult female university students. Arch Gynecol Obstet 279(4):527–532
Fattahi Bafghi A, Yavari M, Hossein Zadeh J (2007) Usefullness of honey from the Islamic point of view and evaluation of its effects on cutaneous leishmaniasis wounds in Balb/C rats invitro. SSU_Journals 14(4):32–36
Sobhanian S, Purahmad M, Modaber M, Pishe SG, Adineh B (2006) Effect of natural honey on diabetic leg sore. J Tehran Fac Med 4(64):108–110
Al-Waili NS (2005) Effects of honey on the urinary total nitrite and prostaglandins concentration. Int Urol Nephrol 37(1):107–111
Al-Waili NS, Boni NS (2003) Natural honey lowers plasma prostaglandin concentrations in normal individuals. J Med Food 6(2):129–133
Mirbagher Ajorpaz N, Hafezi M, Salehi S, Tayebi A, Shenasa F, Zahtabchi S (2012) Comparing the effect of pure and impure honey on severity of pain, amount of bleeding, and duration and interval of menstrual cycles in female students with primary dysmenorrhea. Evid Based Care 2(1):23–33
Direkvand-Moghadam A, Khosravi A (2012) Comparison of verbal multidimensional scoring system (VMS) with visual analogue score (VAS) for evaluating of Shirazi thymus vulgaris on menstrual pain. J Pharm Biomed Sci (JPBMS) 23(23):1–5
Unsal A, Ayranci U, Tozun M, Arslan G, Calik E (2010) Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Upsala J Med Sci 115(2):138–145
Gift AG (1989) Visual analogue scales: measurement of subjective phenomena. Nurs Res 38(5):286–287
Wewers ME, Lowe NK (1990) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 13(4):227–236
Shirvani MA, Motahari-Tabari N, Alipour A (2015) The effect of mefenamic acid and ginger on pain relief in primary dysmenorrhea: a randomized clinical trial. Arch Gynecol Obstet 291(6):1277–1281
Nahid K, Fariborz M, Ataolah G, Solokian S (2009) The effect of an Iranian herbal drug on primary dysmenorrhea: a clinical controlled trial. J Midwifery Women’s Health 54(5):401–404
Acknowledgements
The researchers would like to thank sincerely the participants who without their collaboration the production of this article would be impossible. In addition, our gratitude should be extended to the respectable chancellor, deputy of education and research of the Faculty of Nursing and Midwifery, Research Council and Medical Ethics Council affiliated with Arak University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
LA and TH designed the study, conducted literature research, responsible for data acquisition, and were involved in data analysis. HK were involved in writing the manuscript. LA and BH were responsible for the manuscript drafting and have read and approved the final version.
Corresponding author
Ethics declarations
Sources of support in the form of grants
The study’s research proposal was approved by the research council affiliated with Arak University of Medical Sciences that provided financial support. In addition, this study was registered at the Ethics Committee of Arak University of Medical Sciences that corroborated its ethical considerations (decree number 89-94-3).
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The authors received financial support for the research from Arak University of Medical Sciences and no financial support for publication of this article.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Amiri Farahani, Ë.L., Hasanpoor-Azghdy, S.B., Kasraei, H. et al. Comparison of the effect of honey and mefenamic acid on the severity of pain in women with primary dysmenorrhea. Arch Gynecol Obstet 296, 277–283 (2017). https://doi.org/10.1007/s00404-017-4409-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4409-6