Abstract
Purpose
To investigate the relationship between postmenopausal women’s sclerostin levels and bone density and the factors that may affect this relationship.
Materials and methods
135 postmenopausal patients’ ages, BMIs, hormonal statuses, BMD values, and smoking, and consumption of coffee and dairy products were compared with their sclerostin levels.
Results
No statistical relationship was found between sclerostin level and age in the group with osteoporosis (p = 0.204, r = −0.305). There was a positive, high-level relationship between sclerostin levels and BMI in the osteoporosis group and it was found to be statistically significant (p < 0.001, r = 0.786). No statistical relationship was found between sclerostin level and age in the non-osteoporosis group with (p = 0.496, r = −0.88). There was a positive, moderate relationship between sclerostin levels and BMI in the non-osteoporosis group and it was found to be statistically significant (p < 0.001, r = 0.505). No statistically significant relationship could be found between sclerostin levels and vitamin D (p = 0.723), PTH (p = 0.112), FSH (p = 0.795), E2 (p = 0.627), TSH (p = 0.517), T3 (p = 0.788), and T4 (p = 0.664) blood levels. No significant difference was found among the groups formed by smoking, consumption of coffee and milk, and dairy products, either (p = 0.405; p = 0.626; p = 0.234, respectively). It was monitored that sclerostin’s negative effect observed on BMD scores was independent from age; however, it had a positive correlation with BMI.
Conclusion
As blood sclerostin levels increase, bone mineral density decreases. This negative effect of sclerostin on bone density increases as BMI increases, too. Effects of sclerostin levels on bone density are independent from age, and they are not affect by levels of vitamin D: PTH, FSH, E2 and thyroid hormones, and daily activities, such as smoking and consumption of coffee and milk and dairy products, either.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a disease characterized by weak bone quality in which bone mineral density (BMD) decreases as a result of deterioration in the skeletal microarchitecture and dilution in the bone protein pattern [1]. As bone mineral density and bone resilience decrease, liability to bone fractures increases [2]. It is predicted that there will be 61 million patients under the risk of fracture due to 14 million osteoporosis patients and low bone mass in the United States by 2020 [3].
The approach used today in osteoporosis treatment is the antiresorptive treatment that mitigates bone resorption [4, 5]. In anabolic treatments through which there is an effort to develop to increase bone formation, agents for inhibiting sclerostin are worked on [6, 7].
Sclerostin is a 19-amino-acid glycoprotein which is the product of the SOST gene located on chromosome 17q12–q21 in humans. It is primarily expressed in osteocytes buried in bone matrix and also in cementocytes and mineralized hypertrophic chondrocytes [8]. Sclerostin is an inhibitor indigenous to Wnt signaling. Wnt signaling plays an essential role in osteogenesis and regulation of bone mass. Loss of function in Wnt signaling pathway manifests itself as skeletal fragility and reduced bone mass. Consequently, osteoblastic bone formation is decreased with sclerostin production but increased with sclerostin inhibition [9, 10].
The purpose of this study is to examine the relationship between the bone density and sclerostin levels of patients in the postmenopausal period in which osteoporosis increases and investigate the relationship between sclerostin and patient’s hormonal status and daily life activities.
Materials and methods
This is a cross-sectional study conducted with 135 postmenopausal patients who consulted the Medical School Hospital of Turgut Özal University between April 2015 and March 2016.
The 135 patients had been in menopause for at least 1 year. Those who had history of bone fracture and malignity, who experienced hyperparathyroidism and malabsorption syndrome, had received chemotherapy in the last 5 years, used steroids, and had been on medication that might affect bone metabolism other than bisphosphonate were not included in the study. The patients using antidiabetic agents, antihipertansive drugs, cardiac drugs, asthma drugs, and gastroenterological medication were included in the study as systemic medication use.
Participants’ demographics, such as age, height, weight, body mass index (BMI), and year of menopause, were recorded. Blood samples were taken from the patients under 8-h fasting condition in the morning to perform routine ELISA tests and measure levels of vitamin D, parathormone (PTH), follicular stimulant hormone (FSH), Estradiol (E2), thyroid stimulant hormone (TSH), and T3 and T4. Patients’ chronic diseases, such as diabetes mellitus (DM) and hypertension (HT) and status of systemic administration and bisphosphonate use, were asked. Those who used bisphosphonate were classified in a separate group.
Fasted blood samples were taken from all the patients in the morning for the sclerostin measurement to evaluate them with the ELISA method using the “Sclerostin Eastbiopharm Cat no: CKE90953” kit. The results were given in ng/ml.
Bone density of the patients was measured by Hologic machine using DEXA (Dual Energy X-Ray Absorptiometry) method in L1–L4 bones. The densitometry data, including bone density in lumbar spine and femoral neck, were collected based on the WHO T score values [11]. Accordingly, the patients with lumbar spine and femoral neck T scores of −2.5 and below were accepted as the osteoporosis group.
The patients were asked for their exercise and dietary habits as their daily life parameters. Unfortunately, it was not possible to evaluate the answers statistically, because the exercise habits are not sufficient in the postmenopausal group in our country, and this section was omitted from the study. The patients were classified by their coffee consumption, smoking, and consumption of milk and dairy products, which are deemed to be affecting osteoporosis, as everyday consumption, consumption for 2–3 times a week, less than once a week and non-users.
Approval of Turgut Özal University Ethical Committee and the written consent of all the patients were obtained for this study.
Statistical analysis
All statistical analyses were performed using SPSS for the Windows 11.5 software program (SPSS Inc., Chicago, IL). For the quantitative variables, mean ± sd and median (min–max), for the categorical (qualitative) variables’ percentage (frequency), were used as descriptors in the study. When to look whether there was a statistically significant difference between the categories of a qualitative variable with two categories in terms of a quantitative variable, Student T test was used if the normal distribution assumptions were met; if not, Mann–Whitney U test was used. When to look whether there was a statistically significant difference between the categories of a qualitative variable with three or more categories in terms of a quantitative variable, one-way ANOVA was used if the normal distribution assumptions were met; if not, Kruskall–Wallis test was used. When it is the purpose to see if there was a statistically significant relationship between two quantitative variables, the Spearman’s Rank Correlation Coefficient was used, because at least one of the variables did not meet the normal distribution assumptions. The linear regression was utilized for examining the effect of the quantitative variable(s) on the dependent quantitative variable. Significance level was set at p = 0.05.
Results
Average age of the patients was calculated to be 68.14 ± 9.50, and the median value was calculated to be 69.0 (48.0–89.0). Their BMI average was calculated to be 24.54 ± 2.79, and the median value was calculated to be 25.0 (19.0–30.0). The average levels of blood sclerostin were calculated to be 39.38 ± 46.16 ng/ml, and the median value was calculated to be 23.0 (5.0–238.0) ng/ml.
No statistically significant relationship could be found between sclerostin levels and blood vitamin D (p = 0.723), PTH (p = 0.112), FSH (p = 0.795), E2 (p = 0.627), TSH (p = 0.517), T3 (p = 0.788), and T4 (p = 0.664) levels.
23 of the patients included in the study were accepted to the osteoporosis group (24.5%), and 71 were included in the non-osteoporosis group (74.5%) (41 patients could not be grouped due to data loss.)
Whereas the mean ± sd value of the age of the osteoporosis group was 63.39 ± 9.75, it was 67.96 ± 8.52 in the non-osteoporosis group. A statistically significant difference was found between the osteoporosis and non-osteoporosis groups in terms of age (p = 0.034). Median (min–max) value of BMI in the osteoporosis group was 26.0 (20.0–30.0), while it was 24.0 (19.0–29.0) in the other group. A statistically significant difference was found between the osteoporosis and non-osteoporosis groups in terms of BMI (p < 0.001).
No statistical relationship was found between sclerostin level and age in the group with osteoporosis (p = 0.204, r = −0.305). There was a positive, high-level relationship between sclerostin levels and BMI in the osteoporosis group and it was found to be statistically significant (p < 0.001, r = 0.786). This relationship is charted in Fig. 1.
No statistical relationship was found between sclerostin level and age in the non-osteoporosis group with (p = 0.496, r = −0.88). There was a positive, moderate relationship between sclerostin levels and BMI in the non-osteoporosis group and it was found to be statistically significant (p < 0.001, r = 0.505). This relationship is charted in Fig. 2.
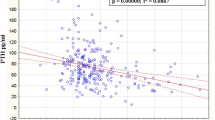
When examining the relationship between sclerostin and BMI, including all the patients, a high-level, positive relationship was found between sclerostin and BMI (p < 0.001, r = −0.688). This relationship is charted in Fig. 3.
As for the relationship between sclerostin and BMD measurement results, a negative and statistically significant relationship was found between all the values and sclerostin. There is a high-level, negative relationship between sclerostin and lumbar spine T and Z scores (p < 0.001, r = −0.645, and p < 0.001, r = −0.650, respectively). A moderate, negative relationship was observed between sclerostin and femoral neck T and Z scores (p < 0.001, r = −0.453 and p < 0.001, r = −0.439, respectively).
No statistically significant difference was found for the sclerostin parameter between the HT and no-HT groups (p = 0.135). The median (min–max) values of sclerostin were found to be 19 (14–113) and 26 (12–238) for the no-HT and HT groups, respectively. Likewise, no statistically significant difference could be found for the sclerostin parameter between the DM and no DM groups (p = 0.884). The median (min–max) values of sclerostin were found to be 24.5 (12.0–238.0) and 23.0 (13.0–225.0) for the no-DM and DM groups, respectively.
There is a significant difference for the sclerostin parameter among the year of menopause groups (p = 0.038). According to the results of the post-hoc test performed to see among which subgroups, there was a difference, and it was found that differences between the group of 20 years and above and the group of 5–10 years (p = 0.012) and the group of 10–20 years and the group of 5–10 years (p = 0.019) caused the significance.
No statistically significant difference was found for the sclerostin parameter between the systemic medication groups (p = 0.485). Similarly, no statistically significant difference was found between the groups that did use and did not use bisphosphonate (p = 0.443).
No significant difference could not be found among the groups formed by smoking, consumption of coffee and milk, and dairy products, either (p = 0.405; p = 0.626; p = 0.234, respectively).
It was seen that the effect of blood sclerostin levels on BMD scores was not found to be related to age.
Sclerostin’s effect on lumbar spine T score was not found to be related to age (p = 0.450). Sclerostin alone explains 26.8% of the change in the lumbar spine T score (p < 0.001). The change of 100 units in sclerostin causes a decrease of 1.6 units in the lumbar spine T score (Lumbar spine T score = −0.264 − 0.016 × sclerostin).
Sclerostin’s effect on lumbar spine Z score was not found to be related to age (p = 0.523). Sclerostin alone explains 28.8% of the change in the lumbar spine Z score (p < 0.001). The change of 100 units in sclerostin causes a decrease of 1.6 units in the lumbar spine Z score (Lumbar spine Z score = −0.050 − 0.016 × sclerostin).
Sclerostin’s effect on the femoral neck T score was not found to be related to age (p = 0,670). Sclerostin alone explains 22.8% of the change in the femoral neck T score (p < 0.001). The change of 100 units in sclerostin causes a decrease of 1.1 units in the femoral neck T score (Femoral neck T score = −0.837–0.011 × sclerostin).
Sclerostin’s effect on femoral neck Z score was not found to be related to age (p = 0.060). Sclerostin alone explains 19.6% of the change in the femoral neck Z score (p < 0.001). The change of 100 units in sclerostin causes a decrease of 0.9 units in the femoral neck Z score (Femoral neck Z score = −1.680 − 0.009 × sclerostin).
When looking up whether BMI, vitamin D, PTH, FSH, E2, and TSH parameters had an impact on the lumbar spine T score, while the sclerostin parameter was along in the model, sclerostin (p = 0.004) along with BMI (p = 0.035) was found to be statistically significant. Sclerostin and BMI explain 33.2% of the change in the lumbar spine T score. The change of 100 units in sclerostin causes a decrease of 0.11 unit in the lumbar spine T score; the change of 10 units in BMI causes a decrease of 1.39 in the lumbar spine T score (lumbar spine T score = 2.108 −0.011 × sclerostin − 0.139 × BMI).
When looking up whether BMI, vitamin D, PTH, FSH, E2, and TSH parameters had an impact on the femoral neck T score, while the sclerostin parameter was along in the model, sclerostin (p = 0.022) along with BMI (p = 0.038) was found to be statistically significant. Sclerostin and BMI explain 28.4% of the change in the femoral neck T score. The change of 100 units in sclerostin causes a decrease of 0.07 unit in the femoral neck T score; the change of ten units in BMI causes a decrease of 1.39 in the femoral neck T score (Femoral neck T score = 0.963–0.007 × sclerostin − 0.103 × BMI).
Discussion
It was seen in this study that bone density decreases as blood sclerostin levels increase in postmenopausal patients. It was revealed that sclerostin levels are not affected by hormonal parameters in blood and dietary habits. It was concluded that blood sclerostin levels are inversely proportional to age and directly proportional to BMI. Sclerostin’s negative effect on bone density was not found to be related to age, and it is in a positive correlation with BMI.
The inhibitor effect of sclerostin on bone mineral density was shown in the study. Studies performed on this subject in the literature are in parallel with negative effects of sclerostin on bone formation via various mechanisms and the fact that sclerostin increases the risk of bone fracture [12–14]. It is thought that therapies to be applied with sclerostin antibodies in osteoporosis treatment will regulate bone formation, and studies are carried out on this subject [4, 5, 10, 14].
According to the results of our study, blood sclerostin levels were not affected by endocrinal parameters. There are publications in the literature that regard bone tissue as an endocrine organ and support that bone formation is affected by hormonal status [15]. Even though no connection could be found in this study, Wang published a paper revealing that FSH increases the risk of osteoporosis [16]. In the study conducted by Liakou et al., it was revealed that blood sclerostin levels do not differ by menstrual cycle. This result coincides with the fact that blood sclerostin levels are not affected by hormonal status [17].
There are studies in the literature, too, showing that there is a negative correlation between blood parathormone level and sclerostin level and blood sclerostin levels change in the vitamin D supplement [18–20]. Difference between previous studies and this study is that those studies evaluated the sclerostin levels after administering PTH and vitamin D externally; however, this study compared the spontaneous blood levels. Moreover, the study group in this research was composed only of menopausal patients; studies in the literature abovementioned also included premenopausal patients. Changes occurring in the bone metabolism with age may be the reason for the difference.
No relationship was found between sclerostin levels and the presence of DM and HT in this study conducted with postmenopausal patients. Yet, there are publications in the literature showing that patients with diabetes have increased levels of sclerostin [21, 22]. There have been articles revealing the relationship between sclerostin and vascular calcification in the literature in recent years; however, as far as the authors are concerned, no studies have been performed to examine the hypertension–sclerostin relationship to date [23–25] (Table 1).
According to the literature, smoking and coffee consumption are risk factors for osteoporosis, and regular consumption of dairy products diminishes the risk of osteoporosis [26–29]. It was aimed in this study to examine the relationship between sclerostin levels and smoking, coffee consumption, and consumption of dairy products, so that it could be understood whether the relationship between these products and osteoporosis is caused by sclerostin, but no relationship could be found. This subject is open for investigation in the literature (Table 1). There is one study in the literature that investigates the relationship between alcohol intake and sclerostin [30].
It has been also reported that bisphosphonate use in postmenopausal osteoporosis increases sclerostin levels [31]. According to the results of this study, no significant difference could be put forth on the subject. This result may be explained by the reason that the patients who participated in the study had different durations of bisphosphonate use and used different preparations.
It was also observed in the study that there is no correlation between blood sclerostin levels and age. Nevertheless, Amrein and Mödder stated in their study performed with premenopausal patients that sclerostin levels increase as individual gets older [32, 33]. In the research conducted by Roforth et al. circulating sclerostin levels and bone mRNA levels of sclerostin were compared using quantitative polymerase chain reaction (QPCR) analyses in needle bone biopsies between the groups of younger individuals and postmenopausal older individuals [34]. As a result, it was seen that blood sclerostin levels in the group of postmenopausal older individuals are higher than in the group of younger individuals, but bone sclerostin mRNA levels do not differ. Despite higher blood sclerostin levels in the group of older individuals than the younger population, this correlation may be over in the postmenopausal group; this may mean what is desired to do with anabolic bone protection treatments is done by the nature itself. The changes observed the sclerostin levels along with age are still a matter of subject open to investigation.
It was seen in the study that blood sclerostin levels are in a positive correlation with BMI and they are effective together in decreased bone density. Similarly, Amrein et al. put forth sclerostin’s positive correlation with BMI [32]. There are studies in the literature which found that sclerostin levels increase or do not change with the weight loss in obese people; however, these data may not be in parallel with the result of these study, because they are related to bariatric surgery and acute weight loss upon a strict diet [35–37]. While a higher percentage of body fat has been associated with elevated sclerostin levels [32] and obese subjects have a much lower bone turnover than healthy controls [38], patients after bariatric sugery also show elevated levels of sclerostin up to 2 years after the initial weight loss [37]. In these cases, high sclerostin levels were negatively associated with bone density loss during weight loss [37]. In addition, higher sclerostin levels have been found in patients suffering from pre-diabetes [39], suggesting that sclerostin may have an impact on insulin action and clearance in humans. No significant difference of sclerostin levels was found in patients diagnosed with diabetes in this study, however.
This is a study that shows the relationship between sclerostin levels and age, BMI, bisphosphonate use, smoking, coffee consumption, and consumption of dairy products in postmenopausal patients. The fact that the study group was composed only of postmenopausal older individuals caused the study to achieve different results than the studies which included younger population. It is obvious with this case that different points of view should be considered for premenopausal and postmenopausal groups. The most important limitation of the study is that all blood measurements were performed in one go by the nature of the cross-sectional structure and number of patients was limited due to data loss. It could have enhanced the value of the study to collect information on patients’ diets and daily life activities rather than relying on their own statements. The literature is in need of prospective studies to be performed for longer years on broader patient series and with more detailed examinations.
In conclusion, bone mineral density decreases as blood sclerostin levels increase. This negative effect of sclerostin on bone density increases as BMI increases, too. Effects of sclerostin levels on bone density are independent from age, and they are not affect by levels of vitamin D; PTH, FSH, E2, and thyroid hormones and daily activities, such as smoking and consumption of coffee and milk and dairy products, either.
Combating obesity needs to become more effective with new treatment modalities which are planned to have an impact on the prevention of osteoporosis through sclerostin.
References
IH N (2001) NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: highlights of the conference. South Med J 94:569–573
Unni S, Yao Y, Milne N, Gunning K, Curtis JR, LaFleur J (2015) An evaluation of clinical risk factors for estimating fracture risk in postmenopausal osteoporosis using an electronic medical record database. Osteoporos Int 26(2):581–587
Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD (2014) Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos 9(1):182
Martin TJ (2014) Bone biology and anabolic therapies for bone: current status and future prospects. J Bone Metab 21(1):8–20
Tella SH, Gallagher JC (2014) Biological agents in management of osteoporosis. Eur J Clin Pharmacol 70(11):1291–1301
van Dinther M, Zhang J, Weidauer SE et al (2013) Anti-sclerostin antibody inhibits internalization of sclerostin and sclerostin-mediated antagonism of Wnt/LRP6 signaling. PLoS One 8(4):e62295
Lin C, Jiang X, Dai Z et al (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24(10):1651–1661
van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW (2005) SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16(3):319–327
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19(2):179–192
Hoeppner LH, Secreto FJ, Westendorf JJ (2009) Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets 13(4):485–496
World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group (meeting held in Rome from 22 to 25 June 1992)
Poole KE, van Bezooijen RL, Loveridge N et al (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD (2013) Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 24(2):489–494
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Chen Q (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588
Fukumoto S, Martin TJ (2009) Bone as an endocrine organ. Trends Endocrinol Metab 20(5):230–236
Wang J, Zhang W, Yu C, Zhang X, Zhang H, Guan Q, Xu J (2015) Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation. PloS One 10(8):e0134986
Liakou CG, Mastorakos G, Makris K et al (2016) Changes of serum sclerostin and Dickkopf-1 levels during the menstrual cycle. A pilot study. Endocrine 54(2):543–551
Mirza FS, Padhi ID, Raisz LG, Lorenzo JA (2010) Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95(4):1991–1997
Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95(11):5056–5062
Dawson-Hughes B, Harris SS, Ceglia L, Palermo NJ (2014) Effect of supplemental vitamin D and calcium on serum sclerostin levels. Eur J Endocrinol 170(4):645–650
García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, Muñoz-Torres M (2011) Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 97(1):234–241
Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, Cataldo D (2012) Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab 97(5):1737–1744
Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E (2015) Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta 438:401–414
Claes KJ, Viaene L, Heye S, Meijers B, d’Haese P, Evenepoel P (2013) Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 98(8):3221–3228
Delanaye P, Cavalier E, Bouquegneau A, Khwaja A (2015) Sclerostin levels in CKD patients: an important, but not definitive, step on the way to clinical use. Kidney Int 88(6):1221–1223
Muftic M, Selimovic EK, Miladinovic K (2013) Osteoporosis-comparative study between quantitative ultrasound of calcaneus and DXA. Med Archives 67(4):289
Oncken C, Prestwood K, Kleppinger A, Wang Y, Cooney J, Raisz L (2006) Impact of smoking cessation on bone mineral density in postmenopausal women. J Women’s Health 15(10):1141–1150
Barrett-Connor E, Chang JC, Edelstein SL (1994) Coffee-associated osteoporosis offset by daily milk consumption: the Rancho Bernardo Study. Jama 271(4):280–283
Lötters, FJB, Lenoir-Wijnkoop I, Fardellone P, Rizzoli R, Rocher E, Poley MJ (2013) Dairy foods and osteoporosis: an example of assessing the health-economic impact of food products. Osteoporos Int 24(1):139–150
González-Reimers E, Martín-González C, De la Vega-Prieto MJ, Pelazas-González R, Fernández-Rodríguez C, López-Prieto J, Santolaria-Fernández F (2013) Serum sclerostin in alcoholics: a pilot study. Alcohol Alcohol 48(3):278–282
Gatti D, Viapiana O, Adami S, Idolazzi L, Fracassi E, Rossini M (2012) Bisphosphonate treatment of postmenopausal osteoporosis is associated with a dose dependent increase in serum sclerostin. Bone 50(3):739–742
Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Fahrleitner-Pammer A (2011) Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 97(1):148–154
Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26(2):373–379
Roforth MM, Fujita K, McGregor UI, Kirmani S, McCready LK, Peterson JM, Khosla S (2014) Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone 59:1–6
Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Villareal DT (2012) Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 27(5):1215–1221
Grethen E, Hill KM, Jones R, Cacucci BM, Gupta CE, Acton A, Peacock M (2012) Serum leptin, parathyroid hormone, 1, 25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J Clin Endocrinol Metab 97(5):1655–1662
Muschitz C, Kocijan R, Marterer C, Nia AR, Muschitz GK, Resch H, Pietschmann P (2014) Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab 100(3):891–901
Ivaska KK, Huovinen V, Soinio M, Hannukainen JC, Saunavaara V, Salminen P, Helmiö M, Parkkola R, Nuutila P, Kiviranta R (2016) Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. doi:10.1016/j.bone.2016.11.001
Daniele G, Winnier D, Mari A, Bruder J, Fourcaudot M, Pengou Z, Tripathy D, Jenkinson C, Folli F (2015) Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care 38(8):1509–1517. doi:10.2337/dc14-2989 (Epub 2015 Jun 17)
Author Contribution
The main author of this study is MNK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MN Kalem declares that she has no conflict of interest. Z Kalem declares that she has no conflict of interest. N Akgun declares that she has no conflict of interest. B Bakırarar declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Financial disclosure
The authors declared that this study has received no financial support.
Rights and permissions
About this article
Cite this article
Kalem, M.N., Kalem, Z., Akgun, N. et al. The relationship between postmenopausal women’s sclerostin levels and their bone density, age, body mass index, hormonal status, and smoking and consumption of coffee and dairy products. Arch Gynecol Obstet 295, 785–793 (2017). https://doi.org/10.1007/s00404-017-4288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4288-x