Abstract
Summary
Sclerostin is a key regulator of bone formation. In a population of 572 postmenopausal women (mean age, 67 years) followed prospectively for a median of 6 years, there was no significant association between baseline levels of serum sclerostin and incidence of all fractures which occurred in 64 subjects.
Introduction
Sclerostin, an osteocyte soluble factor, is a major negative regulator of osteoblastic activity. Circulating sclerostin levels were reported to increase with age and to be modestly associated with bone mineral density (BMD) and bone turnover, but there are no data on the association with fracture risk.
Methods
We investigated 572 postmenopausal women (mean age, 67 ± 8.5 years) from the OFELY population-based cohort. The associations of serum sclerostin measured with a new two-site ELISA and spine and hip BMD by DXA, serum β-isomerized C-terminal crosslinking of type I collagen (CTX), intact N-terminal propeptide of type I collagen (PINP), intact PTH, 25-hydroxyvitamin D [25(OH)D], estradiol, testosterone, and fracture risk were analyzed. At the time of sclerostin measurements, 98 postmenopausal women had prevalent fractures. After a median of 6 years (interquartile range, 5–7 years) follow-up, 64 postmenopausal sustained an incident fracture.
Results
Serum sclerostin correlated positively with spine (r = 0.35, p < 0.0001) and total hip (r = 0.25, <0.0001) BMD. Conversely, serum sclerostin was weakly negatively associated with the bone markers PINP (r = −0.10, p = 0.014) and CTX (r = −0.13, p = 0.0026) and with intact PTH (r = −0.13, p = 0.0064). There was no significant association of serum sclerostin with 25(OH)D, estradiol, free estradiol index, or testosterone. Serum sclerostin considered as a continuous variable or in quartiles was not significantly associated with the risk of prevalent or incident fracture.
Conclusion
Serum sclerostin is weakly correlated with BMD, bone turnover, and PTH in postmenopausal women. It was not significantly associated with the risk of all fractures, although the number of incident fractures recorded may not allow detecting a modest association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteocytes which are terminally differentiated cells embedded within the mineralized matrix play important roles in bone metabolism [1]. These cells which comprise an extensive network of canaliculae are believed to participate in the regulation of the targeted remodeling process by sensing microfractures [1]. This mechanism is crucial for replacing altered bone tissue by a new collagen matrix with optimal biochemical competence. Osteocytes are also important in the termination of the remodeling cycle by secreting factors such as sclerostin which inhibits osteoblast activity and promotes their apoptosis [1].
Sclerostin which is coded by the SOST gene is a member of the DAN (differential screening–selected gene aberrant in neuroblastoma) family of glycoproteins. This family consists of a group of secreted proteins that share the ability to antagonize bone morphogenetic protein (BMP) activity, although sclerostin is not a classical BMP antagonist [2]. Sclerostin binds to the lipoprotein receptor 5 [3], an essential membrane-bound cofactor of the canonical Wnt signaling and, thereby, inhibits this pathway leading to decreased osteoblast activity [4].
The mRNA expression of sclerostin is inhibited by PTH both in vitro [5, 6] and in vivo in mice and rats [7, 8]. Recent studies showed that administration of a monoclonal antibody blocking the activity of sclerostin results in increased of bone mineral density (BMD) and bone strength in ovariectomized rats [9] and primate [10] and an increase of markers of bone formation while decreasing markers of bone resorption in healthy men and women [11].
Because sclerostin is a secreted soluble factor, different immunoassays have been recently developed to measure its concentration in serum, and three are commercially available [12, 13]. Clinical studies performed in healthy individuals showed that serum sclerostin levels increase with age in both women and men, are modestly and inconsistently associated with bone turnover markers and BMD and decrease in postmenopausal women receiving intermittent PTH or 17 β estradiol, whereas bisphosphonate therapy has no effect [14–21]. However, in women with corticosteroid-induced osteoporosis, teriparatide did not decrease sclerostin levels [22]. However, there is a no published study that has investigated the relationships between serum sclerostin and fracture risk assessed prospectively.
The primary objective of this study was to analyze the association of serum sclerostin levels measured by a novel commercially available immunoassay and fracture risk assessed prospectively in a large population of postmenopausal women. We also investigated the association of serum sclerostin with BMD, bone turnover markers, PTH, and steroid hormones.
Material and methods
Subjects
We investigated 572 postmenopausal women (mean age, 67 ± 8.5 years) from the Os des FEmmes de LYon (OFELY) cohort at the ninth annual follow-up. OFELY is an ongoing prospective study of the determinants of bone loss in 1,039 volunteer women, 31 to 89 years of age, recruited between February 1992 and December 1993, randomly selected from the affiliates of a large health insurance company (Mutuelle Générale de l’ Education Nationale) from the Rhône district with an annual follow-up. The OFELY cohort has been described elsewhere [23, 24].
Fracture assessment
Nonvertebral and clinical vertebral fractures were reported during each annual follow-up. For women who did not come to the clinical center, a letter was sent every year to identify the occurrence of fractures. All fractures were confirmed by radiographs or by a surgical report. Only low-trauma fractures (i.e., those occurring as a result of falls from standing height or less) were taken into account, and we excluded fractures of fingers, toes, skull, and face. Vertebral fractures were assessed on lateral X-ray films of the thoracic and lumbar spine obtained every 4 years, including the ninth annual follow-up, in women aged 50 years and over at the inclusion in the study. The semiquantitative method of Genant [25] was used by a trained physician. For the present analysis, prevalent fragility fractures were all those that occurred between the inclusion visit and the ninth annual follow-up, in addition to the fragility fractures of the wrist, humerus, vertebral, or hip that occurred before the inclusion and after the age of 40 years. Incident fragility fractures were all those that occurred between the 9th and the 16th annual follow-up.
Bone mineral densitometry
Areal bone mineral density (BMD) was measured at ninth annual follow-up by dual-energy X-ray absorptiometry (DXA), at the lumbar spine and total hip (QDR 4500A, Hologic®, Waltham, MA, USA). The in vivo precision error of DXA, expressed as the coefficient of variation, was 0.9 and 1 % for lumbar spine and total hip BMD, respectively. A control phantom was scanned every day, and all DXA measurements were performed by the same experienced operator.
Biochemistry
Blood samples were collected between 8:00 and 9:30 a.m. after an overnight fast at the ninth visit of the OFELY which is considered as baseline for this analysis. Serum samples were stored frozen at −70°C until assayed. Serum intact N-terminal propeptide of type I collagen (PINP), and β-isomerized C-terminal crosslinking of type I collagen (CTX) were measured using an automatic test (Elecsys P1NP, Elecsys β–Crosslaps; Roche Diagnostics). Intra- and interassay variations were <8 % for all markers. Serum total 17 β-estradiol was measured by radioimmunoassay without extraction with a limit of detection of 11 pM/L. Bioavailable estradiol was calculated by multiplying the fraction of estradiol not bound to sex hormone-binding globulin with total estradiol. Serum total testosterone was measured by radioimmunoassay after extraction with a limit of detection of 0.04 nM/L. Intra- and interassay variations were <10 % for both dosages. Serum intact PTH was measured by an immunoradiometric assay using two monoclonal antibodies (ELSA-PTH, Cisbio) with a limit of detection of 0.7 pg/mL. Intra- and interassay variations were <8 %. Serum 25-hydroxyvitamin D [25(OH)D] was measured by an automatic competitive two-step chemiluminescence assay (LIAISON 25OH Vitamin D TOTAL, DiaSorin). The lower limit of detection was 4 ng/mL.
Serum sclerostin was measured by a novel two-site ELISA (TECO® Sclerostin EIA Kit) [12]. This assay uses two antibodies raised against human recombinant sclerostin. Briefly, serum samples or standard (human recombinant sclerostin) are incubated with a biotinylated polyclonal antibody as well as with a horseradish peroxidase (HRP)-labeled secondary monoclonal antibody that specifically recognizes human sclerostin in streptavidin-coated wells. After incubation overnight at 2–8°C, the unbound material is washed away. After this washing step, the substrate is added to the well which reacts with the HRP, and color is formed. After a 15-min incubation, the reaction is stopped with HCl, and the plate is read using a plate reader at 450 nm. The amount of color generated is directly proportional to the amount of sclerostin in the sample. In our laboratory, the intra- and interassay coefficients of variation were lower than 10 %. The detection limit defined as the concentration of sclerostin corresponding to the OD value of standard 0 + 3 standard deviations was 0.13 ng/ml. The recovery of spiked standards which was tested by adding three different concentrations of human recombinant sclerostin (0.5, 1, and 2 ng/ml) into eight different serum human samples presenting with various levels of endogenous sclerostin ranged from 88 to 111 %.
Statistical analyses
All data were reported as mean ± SD unless otherwise specified. Association of serum sclerostin with bone turnover markers, BMD, and hormone levels was assessed by linear regression analysis after logarithmic transformation of the data for normalization of the distribution. Association of serum sclerostin with incident fracture was analyzed by logistic regression analyses using sclerostin levels as continuous or categorized (per quartile) variables and were adjusted for age. A sample size of 522 individuals (lower than the size of our population) was needed to detect an odds ratio of 1.6 between incident fracture and serum sclerostin with a power of 0.85 and an alpha risk of 0.05 (two sided).
Results
Baseline associations of sclerostin with bone turnover, BMD, and hormones
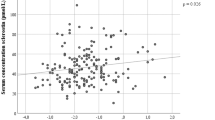
Serum sclerostin was slightly negatively associated with serum PINP and serum CTX, as bone formation and resorption markers, respectively. Serum sclerostin was modestly associated with PTH, but did not correlate with all other hormones including total and free estradiol index, testosterone, and 25(OH)D (Table 1). Serum sclerostin was positively associated with spine and total hip BMD (p < 0.0001, Table 1). Women with levels in the highest quartile of serum sclerostin had spine and total hip BMD values which were 15 and 8.5 % higher, respectively, than individuals with levels in the lowest quartile (Fig. 1).
Serum sclerostin and fracture risk
At baseline, 98 postmenopausal women had a prevalent fracture. During a median of 6 years (interquartile range, 5–7 years) follow-up, 64 postmenopausal women sustained an incident vertebral or non-vertebral fracture. There was no significant association between serum sclerostin levels and prevalent fracture, whether serum sclerostin was considered as a continuous variable (p logistic regression analysis, 0.10) or in quartiles (p Chi-square = 0.35). The proportion of women who had a prevalent fracture was slightly higher in women with levels in the lowest quartile (19.9 %) of sclerostin values than women with levels in the highest quartile (12.6 %), a difference which was not statistically significant after adjustment for age (p = 0.086).
There was also no significant association between baseline sclerostin levels and the incidence of fracture. When considered as a continuous variable, logistic regression analyses showed no significant association between serum sclerostin and fracture incidence (p = 0.22). Table 2 shows the number and percentage of women with incident fracture according to quartiles of baseline serum sclerostin. Consistent with the continuous analysis approach, there was no significant association (p = 0.25) between quartiles of sclerostin and incident fracture risk. Additional analyses showed that serum sclerostin was not associated with prevalent or incident fracture risk when women were classified in tertile or median values. Adjustment for BMD at the spine or hip, prevalent fracture and estrogen use did not have any influence on the association between sclerostin and incident fracture risk.
Discussion
This is the first prospective and large population-based cohort study analyzing the association between serum sclerostin levels and the risk of incident fracture in postmenopausal women. There was no significant association between baseline sclerostin and prevalent or incident fracture risk suggesting that the measurement of this osteocyte factor in the peripheral blood may not have clinical value to predict the risk of all fractures in postmenopausal women with mean age of 67 years.
Most previous studies, but the recent one from Saudi Arabia [20], analyzed the association with BMD and bone turnover markers in small populations. In our large sample, we found that serum sclerostin slightly negatively correlated with the bone turnover markers PINP and CTX, but was positively associated with spine and total hip BMD, which is consistent with a smaller previous study [16]. The counterintuitive positive association of sclerostin with BMD suggests that serum sclerostin may reflect mostly the number of osteocytes—assuming that this number is proportional to level of bone mass—and not the activity of individual cells or at individual bone remodeling units. After adjustment for age, we found that among the various bone-regulating hormones, only intact PTH was significantly negatively associated with serum sclerostin. Such a negative association has been consistently reported in previous studies [15, 20], is in agreement with intervention studies in animal models [5, 7, 26, 27] and in humans [18, 28], and suggests that PTH is probably one of the most important regulators of sclerostin secretion in postmenopausal women. In our large sample of postmenopausal women, we found that serum sclerostin was slightly but not significantly associated with total estradiol levels and the derived free estradiol index. This absence of significant correlation contrasts with previous observational and interventional studies that reported significant negative associations [15, 17, 20]. These discrepancies may be related to differences in population characteristics and/or estradiol assay sensitivity.
Our study has strengths and some limitations. This is the first published study that reports the association between serum sclerostin and the risk of all incident fractures in a large population-based study. This is also one of the largest studies that explores the association of serum sclerostin with two important determinants of bone strength, i.e., BMD and bone turnover markers and several steroid and calcium-regulating hormones. This study has also some limitations. We measured serum sclerostin with a new two-site ELISA that uses antibodies raised against human recombinant protein and which demonstrates adequate precision and accuracy. An internal cross-calibration study performed in a subset of 100 individuals from the present study showed a high correlation (r = 0.90, p < 0.0001) between the assay we used and the other commercial ELISA from Biomedica which has been used in several studies [16, 20, 28] (data not shown). Thus, we believe that the findings would not have been different with the use of the other commercially available test. We analyzed circulating sclerostin levels, whereas this factor is thought to exert primarily its effects locally. Although circulating levels of sclerostin have been shown to be strongly correlated with marrow plasma levels in postmenopausal women [18], it is possible that peripheral levels are not sensitive enough to detect changes occurring locally at the level of the bone. We measured serum sclerostin at a single time point in fasting morning samples. Because there is a diurnal variation of serum sclerostin [29], we cannot preclude that multiple measurements during the day or over several days would have allowed us to detect significant association with fracture risk. Finally, because the number of women with incident fracture was limited, we cannot exclude that we may have found a significant modest association with a larger sample. Indeed, we calculated that our study had at least a 0.85 power (with an alpha of 0.05) to detect an odds ratio of 1.6 of incident fracture among women with increased or decreased serum sclerostin levels. Thus, we were not able to detect a lower association (smaller odds ratio) with a high level of confidence, but probably, a smaller odds ratio would not represent a sufficiently strong association to be of meaningful clinical significance. The relatively low number of fracture also did not allow us to analyze separately vertebral, hip, and non-spine and non-hip fractures. It is thus possible that serum sclerostin may be associated more specifically with one type of fracture as suggested by a recent study reported in an abstract form that found a significant relationship with hip fracture in older women [30].
In summary, this population-based study indicates that although serum sclerostin is modestly correlated with BMD and bone turnover in postmenopausal women, a single measurement of its circulating levels did not predict the risk of all fractures, although our study may lack statistical power to detect a modest association. Circulating sclerostin may however be useful to investigate changes in osteocyte number and/or activity and the effects of agents including bone-forming therapies.
References
Bonewald LF (2011) The amazing osteocytes. J Bone Miner Res 26:229–238
van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CWGM (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887
Poole KE, van Bezooijen RL, Loverridge N et al (2005) Sclerostin is a delayed product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL (2005) Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Vito M, Bonucci E (2007) Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol 38:261–269
Kramer I, Loots GG, Studer A, Keller H, Kneissel M (2009) Parathyroid hormone (PTH) induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588
Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–59
Padhi D, Graham J, Stouch B, Liang F, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26
Mc Nulty M, Singh RJ, Li X, Bergstralh EJ, Kumar R (2011) Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays. Clin Endocrinol Metab 96:E1159–E1162
Costa AG, Cremers S, Rubin MR, McMahon DJ, Sliney J Jr, Lazaret-Castro M, Silverberg SJ, Bilezikian JP (2011) Circulating sclerostin in disorders of parathyroid gland function. J Clin Endocrinol Metab 96:3804–10
Kirmani S, Moedder UK, Hoey K, Peterson J, McCready L, Shreyasee Amin SL, Melton J, Riggs JB, Ralph Muller R, Khosla S (2010) Gender differences in circulating sclerostin levels are established during puberty and correlate with cortical porosity. J Bone Miner Res 21(Suppl 1):S54
Mirza FS, Padhi ID, Raisz LG, Lorenzo JA (2010) Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95:1991–7
Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Lawrence Riggs B, Joseph Melton L 3rd, Khosla S (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379
Mödder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S (2010) Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 26:27–34
Drake MT, Srinivasan R, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
olyzos SA, Anastasolakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E (2011) Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women-the six-month effect of risedronate and teriparatide. Osteoporos Int 23(3):1171–1176
Ardawi MS, Al-Kadi HA, Rouzzi AA, Qari MH (2011) Determinants of serum sclerostin in healthy pre and postmenopausal women. J Bone Miner Res 26:2812–2822
Eastell R, Hannon R, Gossiel F. (2010). Regulators of bone formation in postmenopausal osteoporosis: effect of bisphosphonate treatment. J Bone Miner Res 26(Suppl 1): S31
Gossiel F, Lane N, Eastell R (2011). The effect of glucocorticoid therapy on regulators of bone formation in postmenopausal women treated with PTH. J Bone Miner Res 26(Suppl 1): S80
Arlot ME, Sornay-Rendu E, Garnero P, Vey-Marty B, Delmas PD (1997) Apparent pre- and postmenopausal bone loss evaluated by DXA at different skeletal sites in women: the OFELY cohort. J Bone Miner Res 12:683–690
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Genant HK, Wu CY, Van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller (2007) Control of the SOST enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22:1957–1967
Yu EW, Kumbhani R, Siwila-Sackman E, Leder BZ (2011) Acute decline in serum sclerostin in response to PTH infusion in healthy men. J Clin Endocrin Metab 96:E1848–E1851
Santosh SNH, Joseph F, Hamilton A, Durham B, Robinson A, Tang J, Voras JP, Fraser D (2011) Circulating sclerostin demonstrates a circadian rhythm in young healthy men. J Bone Miner Res 26(Suppl 1): SU389
Arasu A, Xawthon PM Do T, Arora PS, Lui L-Y, Cauley JA, Ebsrud KE, Cummings SR. (2011) Sclerostin and risk of hip fracture in older women. J Bone Miner Res 26:S143
Acknowledgments
We thank Teco Medical for providing ELISA for serum sclerostin.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garnero, P., Sornay-Rendu, E., Munoz, F. et al. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 24, 489–494 (2013). https://doi.org/10.1007/s00198-012-1978-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-1978-x