Abstract
Aims
Single-incision transvaginal mesh for reconstruction of Level I and II prolapses in women with recurrent or advanced prolapse. We evaluated functional, anatomical, sonomorphological and quality-of-life outcome.
Methods
Data were collected retrospectively for preoperative parameters and at follow-up visits. Anatomical cure was assessed with vaginal examination using the ICS-POP-Q system; introital-ultrasound scan for postvoidal residual and description of mesh characteristics was performed. We applied a visual analogue scale (VAS) and the German Pelvic Floor Questionnaire to assess quality-of-life.
Results
Seventy women with cystocele (III: 61.3 %/IV: 16 %), all post-hysterectomy and in majority (81.4 %) after previous cystocele repair, were operated using a single-incision transvaginal technique. Overall anatomical success rate was 95.7 % with significant improvement in quality-of-life (p < 0.0001). Mesh erosion occurred in 5.7 %, one patient presented symptomatic vaginal vault prolapse. Postvoidal residual declined significantly (58 vs. 2.9 %). Sonographic mesh length was 55.7 % of implanted mesh with a wide range of mesh position, but no signs of mesh dislocation. There was no de novo dyspareunia reported, one case of preoperative existing dyspareunia worsened. No severe adverse event was observed.
Conclusions
We hereby present a trial of a high-risk group of patients requiring reconstruction of anterior and apical vaginal wall in mostly recurrent prolapse situation. Our data support the hypothesis of improved anatomical and functional results and less mesh shrinkage caused by the single-incision technique with fixation in sacrospinous ligament in combination with modification in mesh quality compared to former multi-incision techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse is a common condition affecting women with increasing age. Up to 40 % of women aged between 49 and 75 years show evidence of vaginal compartment prolapse including anterior and vaginal vault prolapse [1] leading to approximately 200,000 [2, 3] surgical procedures in the USA per year.
Surgical aims for anterior vaginal prolapse (cystocele) are reconstruction of connective tissue between vaginal wall and bladder. This can be achieved with transvaginal suture plication of connective tissue. In advanced stages of cystocele and recurrent cases often there is no sufficient or only spare connective tissue available leading to recurrence rates of at least 20 % [4, 6, 7], other trials reported numbers as high as 67 % [5].
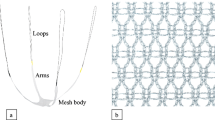
Alternatively, several synthetic mesh implants designed to support the weak native connective tissue can be used to fix anterior wall prolapse. Recent studies [4, 8, 9] demonstrated anatomical success of transvaginal mesh-augmented reconstruction exceeds traditional native tissue repair up to 10 %. On the other hand, mesh-specific complications can lead to re-operation rates that might outweigh the anatomical success rate which led to the FDA security notification in 2011 [10]. A systematic review [11] showed an overall higher re-operation rate in the mesh group compared to traditional suture repair (8.5 vs. 5.8 %). Many questions remain open: the only true mesh-specific symptom is mesh erosion possibly causing infection, bleeding, dyspareunia; the complications in focus such as dyspareunia, pelvic pain, infections or bleeding also occur in patients with native tissue repair. Only rare data are available on the number of complications [12]. Complications after mesh implant might be a result of high mesh load of large and heavy meshes and the surgical technique (e.g. trocar-guided, multi-incision, transobturator approach) [13]. The latest developments of synthetic pre-styled meshes resulted in a change in quality (lower weight) and surgical technique towards single-incision approaches. Amongst the variety of commercial kits available, the mesh used in the present study, Elevate anterior® (AMS) is a pre-styled light-weight (ca. 25 g/m2) macroporous polypropylene mesh with four arms (2 per side) to be fixated in the sacrospinal ligament (proximal arms) and in the obturator membrane (distal arms) addressed to provide a tension-free fixation/stabilisation of the anterior vaginal wall and the vaginal apex [8]. It is a transvaginal single-incision technique able to comply with the requirements of international urogynecologic societies: the usage of monofilament macroporous Type-1 meshes.
It is unclear whether or not the use of mesh implant is justified by the high rate of recurrent anterior vaginal wall prolapse and the benefits of mesh implant for anterior vaginal wall prolapse exceeding mesh-specific complications. Available data are very inhomogeneous and do not help us to distinguish a high-risk population getting benefits out of transvaginal mesh implant. We hereby present data of a homogenous group of presumably high-risk patients to analyse the impact of these developments of transvaginal meshes on anatomical cure rate as well as functional outcome. We wanted to assess complication rates and clarify the benefit of synthetic mesh for patients presenting recurrent cystocele concomitant with vaginal vault prolapse.
Materials and methods
This is a retrospective (non-randomised) study of patients with symptomatic prolapse of anterior vaginal wall, which have been operated in our department in the years 2009/2010 using the Elevate anterior® system (AMS). The majority of patients included presented symptomatic recurrence of cystocele (stage ≥II pelvic organ prolapse quantification system), few patients showed primary advanced stage of prolapse (stage ≥III POP-Q) [14]. Before surgery patients were informed about different options to treat their prolapse, the mesh-specific risks which might lead to re-operation and the need for long-term application of local estrogens. They gave informed consent for surgery and study participation. Approval of local EC (Eth 13/13) is on hand.
One experienced surgeon performed all operations following the protocol for the Elevate anterior®-application [8], including hydrodissection and a full-thickness incision along the anterior vaginal wall (aiming to keep this as short as possible). Preparation of vesico-vaginal space and bilaterally towards the pelvic sidewalls (at the level of the bladder neck up to the inferior ramus of the pubic bone) is followed by detection of ischiatic spines and sweeping off the tissue to isolate the sacrospinous ligament (SSL) without visualising the region. The distal arms of the graft are placed inside the obturator membrane; one absorbable suture is placed to fix the most distal part of the mesh at the level of the bladder neck. The separate apical arms are placed in the SSLs bilaterally approximately 1–2 cm medial to the ischial spine. The apical portion of the graft is then pulled over the arms (approximately 1.5 cm) and the vault adjusted in a tension-free manner and locked with locking eyes. Two absorbable sutures are placed in the apical part of the mesh, after minimal or without vaginal trimming the wall is closed with a running suture.
In the presence of posterior vaginal wall prolapse (rectocele) we performed a native tissue posterior wall repair. We performed concomitant incontinence surgery in women suffering stress urinary incontinence preoperatively (suburethral sling technique). All patients received antibiotic therapy for the duration of their hospitalisation (3 days, local standard of care). Every patient got a transurethral catheter and vaginal packing for 24 h. Before discharge all patients underwent bladder training and a postvoidal ultrasound scan for residual urine.
All patients of our department are routinely invited to a follow-up-visit 2 months after surgery. Preoperative data was collected retrospectively.
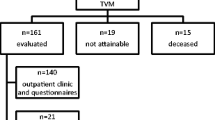
Out of 109 patients operated using the Elevate anterior® system during 2009/2010, we included all patients who met the criteria of being post-hysterectomy. We did not include patients who were operated by the end of 2010 in order to achieve a longer follow-up period. Of 83 patients meeting these criteria, 13 patients could not be reached or refused to come (unknown reasons).
Participants were invited for a follow-up visit 13 months (mean) after surgery; at this time healing was completed and function is supposed to be restored with patients back in daily routine. They were examined by two doctors (not the surgeon). Besides the routine vaginal examination including deep pelvic palpation, the anatomic status was assessed using the ICS-POP-Q system [14].
Patients were regarded as being anatomically cured if they presented POP-Q stage 0 or I.
We routinely use introital and transvaginal ultrasound scan to measure postvoidal residual urine; during follow-up we performed ultrasound examination with empty bladder to evaluate mesh distance in relation to bladder neck, mesh length and mesh position related to pubic bone.
Stress urinary incontinence was evaluated using the Ingelman–Sundberg classification including the cough test.
In cases of preoperatively clinical evident SUI, we performed an urodynamic measurement including cystometry and urethral pressure profile with and without reponation of prolapse (local standard of care).
All patients completed the German Pelvic Floor Questionnaire [15] preoperative and at the follow-up-visit. It is a validated questionnaire to assess symptoms and QoL in patients with pelvic organ prolapse. This questionnaire is divided into four sections: symptoms related to bladder function, bowel function, symptoms of prolapse and sexual function.
We used a visual analogue scale (VAS) to assess patient satisfaction before and after surgery, we asked to answer questions concerning bladder function and bowel function, sexual life impairment and quality-of-life on this scale ranging from 0 (maximum dissatisfied) to 10 (completely satisfied).
We also enquired the achievement of their expectations of surgery (to be indicated between 0 = not at all achieved and 10 = completely achieved).
Data collection and analysis was performed with Excel® (Microsoft Corp.) and SAP R/3®. Statistical analysis of anatomical findings, symptoms and QOL for pre- and post-operative assessment was done using the nonparametric Wilcoxon signed-rank test for differences in medians. In-group analysis was done by using the Mann–Whitney U test. For correlations we applied the Spearman correlation-test.
A p value <0.05 was considered statistically significant.
Results
Majority (n = 63) of the patients have had previous anterior vaginal wall repair and other surgical procedures for POP. n = 7 patients did not have any kind of previous prolapse surgery (including other compartments). Patients’ characteristics are listed in Table 1.
We had no intra- or peri-operative surgical complications. No patient suffered from severe blood loss requiring transfusion. All patients received analgesics following a fixed protocol for the duration of their stay.
There were no cases of delayed recovery; all patients were discharged from the hospital after (median) 3 days. One patient presented persisting pelvic pain after operation with dyspareunia and pain along one arm of the mesh which did not respond to local therapy or physiotherapy resulting in surgical intervention 8 months after the procedure under general anaesthesia (partly transvaginal removal of one mesh arm which resolved the symptoms). One patient had preexisting voiding difficulties (known underlying neuropathy and preoperatively intermittent self-catheterisation) with post-operative urinary tract infection and required a permanent suprapubic catheter.
Four patients (5.7 %) presented erosions. Three of them extended to a maximum size of 3 mm, which resolved after frequent application of local estrogens, no surgical interventions were necessary. One erosion (10 mm) required local excision and application of silver nitrate twice on an outpatient clinic basis in addition to the local oestrogen therapy. Two of these patients did not apply local hormones despite our recommendation. All four of them have had vaginal reconstructive surgery before application of the vaginal mesh.
During vaginal palpation, we discriminated different mesh regions; 21 patients showed discomfort or pain during palpation, usually in one region, which only occurred during follow-up-examination with no impairment in daily life, therefore did not require intervention (Table 2).
Before the operation, 40 (58 %) patients presented postvoidal residual urine >100 ml which decreased significantly (follow-up residual volume >100 ml: n = 2 (2.9 %); p < 0.0001).
Patients with SUI before operation (n = 9) were cured of SUI after concomitant suburethral sling procedure (no signs or symptoms of SUI, negative cough test). Three (4 %) patients developed SUI after mesh repair and were treated with suburethral sling procedure in the meantime.
The number of patients showing symptoms of overactive bladder (OAB) declined significantly from preoperative 53 (75.7 %) to postoperative 21 (30 %) (p < 0.0001); 4 (5.7 %) developed de novo urgency.
While we found significant improvement in the sex score (questions concerning dyspareunia, pain, lubrication, sensations) of the questionnaire (p = 0.0019), the difference in sexual sensations between pre- and post-operative on the VAS was not significant (p = 0.1621).
Thirty-four patients (48.6 %) have been sexually active preoperatively, 29 (41.4 %) postoperatively. However, sexual function after operation remains controversial with 5 (7.1 %) patients stopping sexual activity after surgery (3 because of their partner, 1 because of dyspareunia, 1 with no specification of reason).
Bladder function parameters improved significantly on the VAS and the questionnaire (p < 0.0001), also parameters concerning prolapse-specific symptoms improved significantly (p < 0.0001). 63 women (90 %) were completely satisfied with the operation and would recommend or repeat it if necessary (for further information on subjective outcome, see Tables 3, 4).
Anatomical cure rate (≤stage I, POP-Q) for anterior vaginal wall prolapse was found in 95.8 % (n = 67) (p < 0.0001) (Table 5). Two patients presented with asymptomatic recurrent cystocele stage II without signs of mesh dislocation.
One patient was presenting stage III recurrence of anterior vaginal wall prolapse concomitant with stage III apical prolapse with sonographic signs of mesh dislocation. 32 (45.7 %) patients presented preoperative advanced cystocele with vaginal vault prolapse (stages II–IV), 98 % were anatomically cured (Table 5) after mesh implant.
Patients who have had previous anterior vaginal wall repair had anatomical cure rates of 94.7 % (p < 0.0001). For detailed information see Table 6.
The sonographically measured length of the mesh was 3.9 cm (min 1.7–max 5.5), 55.7 % of the implanted standardised mesh length of 7 cm.
Despite the fixation of the mesh we found a wide range of distance between mesh and bladder neck (mean 1.15 cm, min 0.39–max 2.46). The distance of mesh to bladder neck (>/<1 cm distance) showed no difference in anatomical outcome (p = 0.77). The distance to the bladder neck was not correlated to bladder symptoms in general (bladder score r = −0.014) and showed no correlation to special items of patients satisfaction: symptoms of overactive bladder: r = 0.028, SUI: r = 0.051. Patients with concomitant operative procedures (anterior mesh combined with posterior wall repair) showed no difference in anatomical outcome (p = 0.9414). Patients’ satisfaction after combined surgical procedure did not differ from single anterior and apical wall reconstruction (p = 0.5474).
Discussion
The hereby presented data show excellent anatomical and functional results of transvaginal reconstruction of women with recurrent or advanced prolapse of anterior and apical vaginal compartment using a monofilament Type 1 mesh with single-incision transvaginal technique. These data are consistent with studies of former mesh graft repair [4, 8], but this study differs from the other recently published in terms of design and study population: there is a fundamental heterogeneity of included patients with only a minority of operations for recurrent prolapse situation [8, 16]. This heterogeneity also regards different surgical techniques (combination with hysterectomy, combination with other prolapse procedures) Therefore, current data cannot illuminate the group of patients probably in need for a different surgical approach other than native tissue repair. There is evidence that patients after previous anterior vaginal wall reconstruction are a population which has a poorer outcome with a success rate of only 42.8 % after 2 years, compared to 71.4 % success rate in the group of primary traditional anterior colporrhaphy [6] but there is a lack in standardised procedure for these high-risk patients [17].
The majority (81.4 %) of our study population have had previous native tissue prolapse repair in the same compartment, all of them were post-hysterectomy, and none of them had a concomitant mesh implant. Therefore, we present data that can help to offer a reliable concept: transvaginal mesh implant as a suitable standardised approach for transvaginal reconstruction in recurrent prolapse situation. The latest Cochrane review [4] is advising abdominal/laparoscopic sacropexy as being the gold standard for dominant prolapse of vaginal vault. With one case of recurrence in this study we can approve to this recommendation: this patient was planned for laparoscopic colposacropexy but presented aneurysm of abdominal aorta which forced us to find a consensus decision with the anaesthesiologists and made us switch towards the vaginal approach. Knowing that a single case cannot be a guideline, it is nonetheless useful to gain experiences and rethink indications carefully.
One patient presenting persisting voiding difficulties and residual urine received a suprapubic catheter; before the operation she already suffered from postvoidal residual and routinely used intermittent self-catheterisation, which she refused to continue afterwards.
Former meshes for vaginal reconstruction (Perigee/Prolift anterior) had their limitation in their transobturator fixation: this approach did not provide apical fixation and with good results regarding treatment of cystocele there was a lack in correcting vaginal vault instability/prolapse [6, 18, 19]. De Tayrac et al. described the lack of sufficient support of the vaginal vault, which can lead to anatomical failure [19, 20]. They also claimed dyspareunia being a result of mesh shifting caused by the lack of apical fixation [19].
McLennan et al. [28] recently published data of a perioperative comparison of Prolift® and Elevate® transvaginal mesh procedures with similar outcome, especially no difference in parameters such as analgesics and pain scores. It is again inhomogeneous in type of surgery and study population with a lower pain score after three-compartment reconstruction in the Elevate® arm. We consider the changes made in developing the Elevate® mesh in terms of mesh quality and surgical technique as improvements which might be beneficial for patients. Therefore, we not only performed a vaginal examination on follow-up, but also a thorough introital ultrasound to get more information on the mesh.
In comparison to the transobturator technique on sonomorphological findings [18, 20, 21], we found a larger part of vagina covered by the mesh. 55 % of the implanted mesh was measured at follow-up exam, in former studies conducted in our centre the Perigee and Prolift meshes showed a length after implantation of 43 %. The apical fixation seems to prevent the mesh from shrinking in comparison to transobturator fixation techniques.
We assume that fixation of proximal arms in sacrospinal ligaments provides a more stable mesh length covering a larger part of vaginal tissue and insusceptible to cicatrisation.
The mesh shrinkage does not only seem to be due to operation technique, but also to an inflammatory reaction with connective tissue which cannot be fully prevented and is well known from observations in the abdominal wall. The observations of Klosterhalfen et al. [22, 23] on mesh reaction also lead to the conclusion of better outcome with light-weight, macroporous material.
The use of additional sutures to fixate the mesh remains controversial: our hypothesis of reduced bladder function (bladder outlet obstruction) after additional fixation sutures in the bladder neck region is challenged since we could not find a higher number of functional difficulties after this additional suture. On the other hand, the sutures do not entirely keep the mesh in place. Despite our standardised surgical method the distance of bladder neck to the distal part of the mesh showed a surprisingly large variety (0.39–2.46 cm). Since we do not have a group to compare, we cannot tell the impact of fixation sutures on mesh position. The distance to bladder neck does not seem to make a difference in subjective or objective outcome.
In recent studies, mesh exposure is the complication contributing to majority of surgical revisions, its number of occurrence varies, with a rate of about 10 % in the Cochrane update [4]. The learning curve and surgical skills are discussed to be related to mesh-erosion rates as well as mesh quality [22–24] and operation technique. These factors may contribute to the wide-ranging number of occurrence of mesh exposure.
Assuming that patients with recurrent prolapses have a lower tissue quality (due to scar tissue after operation and presumably lower quality of connective tissue being a reason for recurrence), we had a low rate of erosion. This low number is in line with the findings of the Nordic transvaginal mesh group [25] who described a re-operation rate for mesh exposure of 3.2 %. We suspect two main factors for this low erosion rate: one is the mesh quality itself resulting in a lower mesh load. The other factor is supposed to be the surgical technique. Murphy et al. [12] in their answer to the 2011 FDA conversation concerning the potential risks of transvaginal mesh highlighted the surgical technique and the experience of the surgeon to be an important factor in minimising the complication rate, especially the rate of exposure. Trials performed by experienced surgeons (who are performing this procedure on a regular basis) showed lower erosion rates [12, 25, 26]. The further development of meshes into single-incision methods in contrast to former transobturator meshes is consistent with our low rate of complications. The hydrodissection of the area to be operated on reduces bleeding and therefore reduces infection, defect healing and exposure by reduction of hematoma formation. Additionally, the full-layer incision allows covering the implant with a thick layer of vaginal epithelium. However, this aspect remains controversial. Currently, there are no data comparing mesh-erosion rates after full-layer incision and dissection between the smooth muscle layer of vaginal wall and endopelvic fascia. This is a crucial aspect since frequently there is a lack of “normal” anatomical structures especially the endopelvic fascia in patients with recurrent cystoceles.
Doubtless the tissue support of mesh implant leads to improved anatomical results and presumably prevents prolapse recurrences and will therefore probably stay as one component in surgical therapy for vaginal prolapse. Gynaecologic surgeons need to focus on the management of complications. Firoozi et al. [27] are highlighting the need for a standardised management and reporting system for the complications that will occur with the persisting and/or rising number of transvaginal mesh graft intervention. According to the experiences in other trials [12], the majority of erosions (as a mesh-specific complication) in our trial could as well be treated in an outpatient clinic setting. In our study, only one patient required surgery under general anaesthesia to remove a part of the mesh arm.
The quality-of-life and subjective parameters improved significantly in all comparisons concerning anterior vaginal wall symptoms as bladder-emptying, sensation of prolapse.
Especially the latest Cochrane analysis focused on the fact that quality-of-life parameters improved independently of anatomical outcome and surgical procedure [4]. This study cannot give an answer to this question since we do not have a group to compare. This dilemma highlights the fact that we need to have more information on the special population presumably having a poorer outcome with symptomatic prolapse recurrences.
This leads to the main limitation of our study: its retrospective one-arm design. Our data can only be descriptive.
The concerns of the FDA published in July 2011 [10] about potential risks and complications of mesh implants caused a critical dispute leading to more innovations and developments in pelvic organ prolapse surgery. We understand this technique to be one tool in the management of POP. We still need to focus on specifying the group of patients gaining the highest benefit out of this procedure. However, it is not useful to generally criticise all transvaginal mesh applications. The latest development of meshes including modification of surgical technique into single incision, standardised bilateral apical fixation is a step forward and provides an important component of the operative spectrum of gynaecological prolapse surgery, especially for women after native tissue fascial reconstruction with recurrent disease. Further discussions have to discriminate between (so far) short-term data of latest developments and long-term data of longer known synthetic materials before giving general advices.
Conclusions
We hereby present a trial of a homogenous group of patients requiring reconstruction of anterior and apical vaginal wall, in majority in recurrent prolapse situation. Our data support the assumption of improved anatomical and functional results after transvaginal mesh reconstruction indicating this technique being appropriate for women in recurrent disease. Less mesh shrinkage and reduction of resulting complications might be caused by transvaginal single-incision technique with fixation in sacrospinous ligament in combination with modification in mesh quality compared to former transvaginal multi-incision techniques.
References
Nygaard I, Barber MD, Burgio KL (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300:1311–1316
Jelovsek JE, Maher C, Barber MD (2007) Pelvic organ prolapse. Lancet 369(9566):1027–1038 (review)
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89(4):501–506
Maher CM, Feiner B, Baessler K, Glazener CM (2011) Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J 22(11):1445–1457
Maher CM, Baessler K (2006) Surgical management of anterior vaginal wall prolapse. Urogyn J Pelvic Floor Dysfunct 17(2):195–201 (review)
Peterson TV, Karp DR, Aguilar VC, Davila GW (2010) Primary versus recurrent prolapse surgery: differences in outcomes. Int Urogynecol J 21(4):483–488
Whiteside JL, Weber AM, Meyn LA, Walters MD (2004) Risk factors for prolapse recurrence after vaginal repair. Am J Obstet Gynecol 191(5):1533–1538
Moore RD, Mitchell GK, Miklos JR (2012) Single-incision vaginal approach to treat cystocele and vault prolapse with an anterior wall mesh anchored apically to the sacrospinous ligaments. Int Urogynecol J 23(1):85–91
Moore RD, Miklos JR (2009) Vaginal repair of cystocele with anterior wall mesh via transobturator route: efficacy and complications with up to 3-year follow up. Adv Urol, 743831
FDA-Safety Communication (2011) UPDATE on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse (alerts and notices, medical devices)
Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE (2009) Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol 113(2 Pt 1):367–373
Murphy M, Holzberg A, van Raalte H, Kohli N, Goldman HB, Lucente V, Pelvic Surgeons Network (2012) Time to rethink: an evidence-based response from pelvic surgeons on the FDA safety communication: update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Int Urogynecol J 23(1):5–9
Moore RD, Lukban JC (2012) Comparison of vaginal mesh extrusion rates between a lightweight type I polypropylene mesh versus heavier mesh in the treatment of pelvic organ prolapse. Int Urogynecol J 23:1379–1386
Hall AF, Theofrastous JP, Cundiff GW, Harris RL, Hamilton LF, Swift SE, Bump RC (1996) Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. Am J Obstet Gynecol 175(6):1467–1470
Baessler K, Junginger B (2011) Validation of a pelvic floor questionnaire with improvement and satisfaction scales to assess symptom severity, bothersomeness and quality of life before and after pelvic floor therapy. Aktuelle Urol. 42(5):316–322
Azaïs H, Charles CJ, Delporte P, Debodinance P (2012) Prolapse repair using the Elevate™ kit: prospective study on 70 patients. Int Urogynecol J 23:1421–1428
Shippey S, Gutman RE, Quiroz LH, Handa VL (2008) Contemporary approaches to cystocele repair: a survey of AUGS members. J Reprod Med 53(11):832–836
Feiner B, Maher C (2010) Vaginal mesh contraction: definition, clinical presentation and management. Obstet Gynecol 115:325–330
De Tayrac R, Deffieux X, Gervaise A, Chauveaud-Lambling A, Fernandez H (2006) Long-term anatomical and functional assessment of trans-vaginal cystocele repair using a tension-free polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct 17(5):483–488
Velemir L, Amblard J, Fatton B, Savary D, Jacquetin B (2010) Transvaginal mesh repair of anterior and posterior vaginal wall prolapse: a clinical and ultrasonographic study. Ultrasound Obstet Gynecol 35(4):474–480
Tunn R, Picot A, Marschke J, Gauruder-Burmester A (2007) Sonomorphological evaluation of polypropylene mesh implants after vaginal mesh repair in women with cystocele or rectocele. Ultrasound Obstet Gynecol 29(4):449–452
Klinge U, Klosterhalfen B, Müller M, Schumpelick V (1999) Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165(7):665–673
Klinge U, Junge K, Stumpf M, Klosterhalfen B (2002) Functional and morphological evaluation of a low-weight, monofilament polypropylene mesh for hernia repair. J Biomed Mater Res 63(2):129–136
Kapoor DS, Nemcova M, Pantazis K, Brockman P, Bombieri L, Freeman RM (2010) Reoperation rate for traditional anterior vaginal repair: analysis of 207 cases with a median 4-year follow-up. Int Urogynecol J 21(1):27–31
Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C, Nordic Transvaginal Mesh Group (2011) Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 364(19):1826–1836
Long CY, Lo TS, Wang CL, Wu CH, Liu CM, Su JH (2012) Risk factors of surgical failure following transvaginal mesh repair for the treatment of pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 161(2):224–227
Firoozi F, Ingber MS, Moore CK, Vasavada SP, Rackley RR, Goldman HB (2012) Purely transvaginal/perineal management of complications from commercial prolapse kits using a new protheses/grafts complication classification system. J Urol 187(5):1674–1679
McLennan GP, Sirls LT, Killinger KA, Nikolavski D, Boura JA, Fischer MC, Peters KM (2013) Perioperative experience of pelvic organ prolapse with the Prolift® and Elevate® vaginal mesh procedures. Int Urogynecol J 24:287–294
Conflict of interest
Hengst L, Schwertner-Tiepelmann N: none.
Marschke J and Beilecke K: speaker’s honoraria from AMS, Sub-Investigators in a trial conducted by AMS.
Tunn R: speaker’s honorarium from AMS, Investigator in a trial conducted by AMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marschke, J., Hengst, L., Schwertner-Tiepelmann, N. et al. Transvaginal single-incision mesh reconstruction for recurrent or advanced anterior vaginal wall prolapse. Arch Gynecol Obstet 291, 1081–1087 (2015). https://doi.org/10.1007/s00404-014-3497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3497-9