Abstract
Purpose
The objective of this study is to quantify and evaluate the expression of several important proteins in TGF-β1/Smad pathway in the anterior vaginal wall in postpartum rats with stress urinary incontinence (SUI).
Methods
Forty 8-week-old Sprague–Dawley (SD) female rats were randomized into three groups: blank group (n = 10), control group (n = 10) and SUI group (n = 20). Rats in blank group were non-pregnant, while rats in the control and SUI groups underwent normal parturition and normal parturition plus immediate postpartum vaginal balloon dilation, respectively. 1 week after dilation, a sneezing experiment and pad test were performed and the anterior vaginal wall was collected. The histological changes of the anterior vaginal wall were assessed by hematoxylin–eosin (HE) staining, and the expression of TβR-2, Smad3 and Smad7 in the anterior vaginal wall was detected by immunohistochemical staining and Western blotting.
Results
HE staining showed that collagen was more fragmented, sparse and disorganized in the SUI group compared with the control and blank groups. Compared with the blank group, the expression of TβR-2 and Smad7 protein was significantly increased in the vaginal anterior wall in the control and SUI groups (P < 0.05), while their levels in the SUI group were significantly higher than those in the control group (P < 0.05). Expression of Smad3 protein in the anterior vaginal wall of SUI rats was significantly decreased compared with the blank and control groups (P < 0.05).
Conclusion
Dysregulation of the TGF-β1/Smad signaling pathway may involve in the pathogenesis of SUI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) is the most common type of urinary incontinence in women. It is characterized by involuntary leakage of urine during activities that increase intra-abdominal pressure, such as coughing, sneezing and lifting. A recent survey from 328 women reported that 25.9 % of women appear with SUI complication within 12 months after their first delivery [1]. It has been demonstrated that the prevalence of SUI is highly associated with pregnancy, vaginal delivery, connective tissue disease and other factors [2]. Vaginal delivery usually causes neurologic damage of the pelvic floor and direct injury to muscle and connective tissue. SUI is usually caused by loss of support of the urethra, which is usually a consequence of damage to the pelvic support structures due to parturition.
Collagen is an important component in extracellular matrix (ECM) and plays a critical role in maintaining the normal functions of pelvic support structure. Previous studies have demonstrated that incontinence is associated with the reduced content of collagen type I or III [3, 4]. Dysfunction of collagen in pelvic support structure results in reduced tension and elasticity, which is one of the major pathogeneses for SUI. Transforming growth factor β (TGF-β) is a pleiotropic cytokine, which plays important roles in many physiological and pathological processes [5, 6]. The canonical TGF-β1 pathway includes a few key steps: (1) active TGF-β1 binds to and activates TGF-β receptor II (TβR-2); (2) TβR-2 recruits and activates TβR-1; (3) TβR-1 phosphorylates Smad2 and Smad3; (4) phosphorylated Smad2/Smad3 complex binds to Smad4 and enters the nucleus to regulate the expression target genes [5, 6]. However, Smad7 is featured as an important Smad inhibitor, which associates stably with TβR-1 and blocks phosphorylation of Smad2/Smad3 [7]. It has been demonstrated that TGF-β signaling plays an important role in the regulation of collagen genes, such as COL1A1 and COL1A2 [8–11]. Especially, Smad3 is likely to play an important role in the stimulation of COL1A2 promoter activity elicited by TGF-β [11]. Moreover, a recent study reported that collagen I/III and reticular fibers were also significantly decreased and TGF-β signaling was activated in SUI rats [12]. Thus, previous evidence indicates that TGF-β1 may be involved in the pathogenesis of SUI through regulating collagen synthesis.
Therefore, in this study, we examined the change in collagen deposition and investigated the expression of TβR-2, Smad3 and Smad7 in the anterior vaginal wall of SUI rats compared with that in rats from the blank and control groups.
Materials and methods
Animals
This study was approved by the Institutional Animal Care and Ethics Committee of Guangzhou Medical University. Forty 8-week-old healthy female SD rats were provided by the Experimental Animal Center of Guangdong Province, and randomized into three groups: blank group (n = 10), control group (n = 10) and SUI group (n = 20). Rats in the blank group were non-pregnant without any treatment, while rats in the control and SUI groups underwent normal parturition and normal parturition plus immediate postpartum vaginal balloon dilation, respectively. Vaginal balloon dilation was performed as previously described [13]. Briefly, a urethral catheter (18F) was placed into the vagina and then the balloon was filled with 3 ml water. A 130-g weight was placed on the catheter to provide constant pull pressure to the pelvic floor for 6 h. 1 week after dilation, a sneezing experiment and pad test were executed, details in “Evaluation of SUI model”.
Evaluation of SUI model
The SUI model was assessed by a sneezing experiment and pad test. For sneezing experiments, the rats were intraperitoneally anesthetized by 10 % chloral hydrate (0.03 ml/kg) and the bladder was emptied by an epidural catheter. The maximum bladder capacity was measured by filling the bladder with methylene blue dissolved in sterile saline until the first drop of urine leakage outside the external orifice of the urethra was observed. Then, the bladder was emptied again and filled with a volume of methylene blue solution equaling half of the maximum bladder capacity. The sneezing reflex was induced by inserting a piece of severed rat’s beard into the nostril to increase abdomen pressure. The sneeze test was conducted twice consecutively; if any amount of methylene blue outflow was observed from the external meatus, the sneezing experiment was considered positive [14, 15]. For the pad tests, a piece of gauze (length approximately 4 cm, width approximately 2 cm) was used as a urinal pad, then the urine pad was weight with a scale and sewed onto the periurethral skin. When the animals were recovered after anesthesia, the bladder was emptied again and intragastric administration of water was carried out (volume 1 ml/100 g). The rats were released for free activity, and the pad weight was measured after 1 h. If the pad weight had increased, the pad test was considered positive; otherwise, the test was considered negative. Rats with a positive sneezing experiment and pad test [16] were considered as a successful SUI model.
Histological examination
The anterior vaginal tissues were harvested. The upper half of the samples were fixed in neutral buffered formalin for 12 h, embedded in paraffin, and cut into 5-μm-thick sections. The sections were stained with hematoxylin–eosin (HE) following standard procedures [17]. The other half of the samples was used for western blot analysis.
Immunohistochemistry
Tissue sections were deparaffinized and hydrated consecutively in 95, 85, and 75 % ethanol. Antigen retrieval was carried out by boiling sections in 0.1 M citrate buffer for 15 min. To quench endogenous peroxidase, sections were incubated with 3 % w/w hydrogen peroxide in methanol at room temperature for 10 min, then incubated with antiserum for 1 h. The sections were then incubated with primary antibodies against TβR-2 (1:100 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Smad3 (1:100 dilution; Abcam, Cambridge, MA), or Smad7 (1:100 dilution; Abcam) at 4 °C overnight. The following steps were performed using an HRP-polymer anti-rabbit IHC Kit (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) following the manufacturer’s instructions. The sections were incubated with secondary antibody (1:200 dilution) for 1 h, and the signals were developed by diaminobenzidine (DAB) staining. The staining results were evaluated by two pathologists who were blinded to this study. Immunochemical scoring was given based on the percentage of positive cells and the intensity of staining in five random fields under an optical microscope. The intensity of staining was scored as: 0, no staining; 1, straw yellow; 2, brown; and 3, dark brown; The percentage of positive cells was scored as: 0, 0 %; 1, 1–25 %; 2, 26–50 %; 3, 51–75 %; or 4, 76–100 %. The final score was the sum of intensity and percentage scores. Slides with a final score ≤3 were considered negative, while those with a final score >3 were considered positive.
Western blot analysis
Vaginal tissues were homogenized and lysed in radio immune-precipitation assay (RIPA) buffer supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF) (Bioteke Corporation, China) for 1 h, and centrifuged at 15,000 rpm for 30 min at 4 °C. The protein concentration was measured using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). 40 µg/lane of each protein was separated on 10 % sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and then transferred to cellulose acetate membranes (Millipore, Bedford, MA). The membranes were blocked with 5 % nonfat dry milk in Tween/Tris-buffered solution (TTBS) for 1 h at room temperature, followed by incubation with rabbit anti-TβR-2 (1:500), anti-Smad3 (1:2,000), anti-Smad7 (1:500), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:3,000; Proteintech Group, Hangzhou, China) antibodies overnight at 4 °C. The membranes were washed with TBST three times and incubated with HRP-conjugated anti-rabbit IgG (1:5,000, Jackson) for 1 h at room temperature. Proteins were detected by enhanced chemiluminescence (Thermo Scientific, Tewksbury, MA) and analyzed by Image J software.
Statistical analysis
Statistical analysis was performed using SPSS13.0 package (Chicago, IL). Data were presented as the mean ± standard deviation (SD). Differences among groups for ranked data were analyzed by Fisher exact test. Continuous variables were compared by analysis of variance (ANOVA), and multiple comparisons were analyzed by the least significant difference (LSD) test. P < 0.05 was considered statistically significant.
Results
Establishment of SUI model
According to the results of the sneezing experiment and pad test, 10 of 19 rats in the SUI group were verified as successful SUI models (Table 1).
Histological changes in the anterior vaginal tissues
Histology of the anterior vaginal tissue was assessed by HE staining. We found that the collagen fibers in the anterior vaginal wall of blank rats were long, well organized and tightly arranged bundles (Fig. 1a, b), while the collagen fibers in rats after natural delivery (control group) showed discontinuous and disorganized structure (Fig. 1c, d). Compared with blank and control rats, the collagen fibers in the SUI rats displayed more fragmented, sparse and disorganized structure (Fig. 1e, f). However, there was no obvious difference in the number of collagen fibers in the anterior vaginal wall between blank and control groups (Fig. 1a, c).
HE staining of the anterior vaginal tissues. a, b Blank group, the collagen fibers in the anterior vaginal wall are well arranged and tight in a bundle. c, d Control group, the collagen fibers are not neat and organized. e, f SUI group, the collagen fibers are obviously disorganized, broken and reduced. a, c and e ×100 magnification; b, d and f ×400 magnification
Expression of TβR-2, Smad3 and Smad7 in the anterior vaginal wall
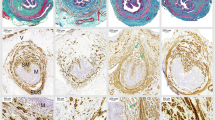
Expression of TβR-2, Smad3 and Smad7 in the anterior vaginal wall was examined by immunohistochemical staining and Western blotting. We found that TβR-2 was mainly detected in the cytoplasm and cell membrane of fibroblasts and epithelial cells (Fig. 2a–c). Quantitation of TβR-2 staining showed that TβR-2 expression was obviously elevated in the control and SUI groups (Table 2). Consistent with this result, western blot results verified that the TβR-2 level was significantly upregulated in the anterior vaginal wall of control rats compared with that in blank rats (P < 0.05), while the TβR-2 level in the anterior vaginal wall of SUI rats was significantly higher than those in blank and control rats (P < 0.05, Fig. 3a, b). Positive Smad3 was observed in the cytoplasm and nucleus of fibroblasts, epithelial cells and smooth muscle cells of the blank and control groups (Fig. 2d, e). The results from Smad3 scoring and western blot analysis showed that Smad3 expression was significantly decreased in the anterior vaginal wall of control rats compared with blank rats and dramatically reduced in the anterior vaginal wall of SUI rats compared with blank and control rats (P < 0.05, Table 2; Fig. 3a, b). Positive Smad7 was localized in the nuclei and cytoplasm of fibroblasts, epithelium cells and smooth muscle cells of the control and SUI groups (Fig. 2h, i). Quantification of Smad7 expression demonstrated that the Smad7 level in the anterior vaginal wall of control rats was significantly higher than that in blank rats, while its level in the anterior vaginal wall of SUI rats was significantly upregulated compared with blank and control rats (P < 0.05, Table 2; Fig. 3a, b).
Representative graphs for TβR-2, Smad3 and Smad7 staining in the anterior vaginal wall tissue. a, d, g SUI rats; b, e, h control rats after delivery; and c, f, i blank rats without treatment. a, b, c TβR-2 is located in the cytoplasm or on the cell membrane of fibroblasts and epithelial cells. d, e, f Smad3-immunoreactive signals are detected in cytoplasm and nucleus of fibroblasts, epithelial cells and smooth muscle cells. g, h, i Positive Smad7 is localized in the nuclei and cytoplasm of fibroblasts, epithelial cells and smooth muscle cells
Quantitative detection of TβR-2, Smad3 and Smad7 in the anterior vaginal wall. a Representative blots for TβR-2, Smad3 and Smad7 in the anterior vaginal wall tissues. b Quantitation of TβR-2, Smad3 and Smad7 expression. Relative expression of TβR-2, Smad3 and Smad7 is determined by the ratio to GAPDH expression. Different letters on the top of column represents different significance. P a < 0.05, which shows there is a significant difference for expression of Smad3, Smad7 and TβR-II in vaginal walls in SUI group compared with control group; P b < 0.05, that is to say difference for expression level of Smad3, Smad7 and TβR-II in vaginal walls is detected in control group compared with blank group; P c < 0.05, which shows there is a significant difference for expression of Smad3, Smad7 and TβR-II in vaginal walls in SUI group compared with blank group
Discussion
It has been demonstrated that the pathogenesis of SUI is highly associated with the dysfunction of ECM [18, 19]. Collagen is one of the most important elements in ECM; collagen types I and III play an important role in maintaining tissue tensile strength and the mechanical stability of the pelvic support structure. Previous studies revealed that the content of collagen types I and III in the periurethral connective tissue of SUI patients is markedly decreased compared to that in patients without SUI [20, 21]. In the present study, we observed that the collagen fibers in the anterior vaginal wall of control and SUI rats were dramatically reduced compared with non-pregnant blank rats. Moreover, the collagen fibers in the anterior vaginal wall of SUI rats were more fragmented, sparse and disorganized. These findings are consistent with previous studies [20, 21], and further support the hypothesis that excessive squeezing to the vagina during parturition has obvious damage to vaginal connective tissues, especially collagen fibers, resulting in SUI.
TGF-β1 plays an important role in the regulation of collage expression in cells and ECM. A previous study demonstrated that TGF-β1 activity in pelvic connective tissue was regulated by cyclic reproductive hormones in women with SUI [22]. The same group found that the levels of total and active TGF-β1 in the ECM isolated from SUI vaginal fibroblasts were negatively regulated by relaxin [23]. Moreover, Li et al. [12] reported that the expression of TGF-β1, MMP-9, and phosphorylated Smad2 (p-Smad2) was significantly elevated in the urethral tissues of SUI rats. Therefore, we hypothesize that TGF-β1 signaling may be involved in the pathogenesis of SUI. However, the underlying mechanism is still unclear.
TβR-2 and Smad3 are two important elements in the canonical TGF-β1 signaling pathway, while Smad7 serves as a TGF-β signaling inhibitor by inhibiting TβR-1 activity [5, 6]. In this study, we found that the expression of TβR-2 and Smad7 was obviously upregulated in the anterior vaginal wall of control rats and this effect was more dramatic in SUI rats. Interestingly, the expression of Smad3 in the anterior vaginal wall showed an opposite trend as that of TβR-2 and Smad7. However, the molecular mechanisms regarding upregulated expression of TβR-2 and Smad7, or downregulated expression of Smad3, were not addressed in this study and, therefore, need further study. Our data indicate that low expression of Smad3 and high expression of Smad7 in the anterior vaginal wall will inhibit the TGF-β1/Smad signaling, which may reduce collagen deposit in the anterior vaginal wall. Moreover, the expression of TβR-2, Smad3 and Smad7 in the anterior vaginal wall in control rats was also significantly upregulated compared with that in blank rats. These data support the hypothesis that excessive squeezing to the vagina during parturition may activate TGF-β/Smad3 signaling, an effect that is strengthened by postpartum vaginal dilation. It has been demonstrated that the disruption of the TGF-β/Smad signaling pathway has a protective effect on chronic tissue inflammation and fibrosis in different diseases, such as chronic obstructive pulmonary disease [24], intestinal fibrosis [25], liver fibrosis [26] and renal fibrosis [27]. Moreover, previous studies revealed that deletion of Smad3, but not Smad2, inhibits fibrosis in different diseases [28, 29]. Thus, in this study, the reduced Smad3 in the anterior vaginal wall of SUI rats may lead to the disruption of collagen fibers.
Although TGF-β/Smad signaling in the urethral tissues is found to link with the pathogenesis of SUI [12], the role of TGF-β/Smad signaling, especially TβR-2, Smad3 and Smad7, in the anterior vaginal wall has not been clarified. Thus, this study provides preliminary evidence for the potential role of TGF-β/Smad3 signaling in the anterior vaginal wall of an SUI model. However, further investigation is needed to study the expression and role of TβR-2, Smad3 and Smad7 in the urethral tissues, which will provide direct evidence for the clarification of SUI molecular mechanisms. Additionally, it will be interesting to investigate the reasons for the differential expression of TGF-β/Smad3 signaling elements in the SUI model.
In summary, in this study, we demonstrated that the expression of TβR-2 and Smad7 was significantly increased in the anterior vaginal wall of SUI rats compared with that in control and blank rats, while Smad3 expression was dramatically reduced. The collagen fibers in the anterior vaginal wall of SUI rats were obviously impaired compared to control and blank rats. These data suggest that the TGF-β/Smad3 signaling may be involved in the pathogenesis of SUI.
Abbreviations
- SUI:
-
Stress urinary incontinence
- IHC:
-
Immunohistochemistry
- ECM:
-
Extracellular matrix
- TGF-β1:
-
Transforming growth factor-β1
- TβR-2:
-
Transforming growth factor receptor II
References
Chan SS, Cheung RY, Yiu KW, Lee LL, Chung TK (2013) Prevalence of urinary and fecal incontinence in Chinese women during and after their first pregnancy. Int Urogynecol J 24(9):1473–1479. doi:10.1007/s00192-012-2004-8
Findik RB, Unluer AN, Sahin E, Bozkurt OF, Karakaya J, Unsal A (2012) Urinary incontinence in women and its relation with pregnancy, mode of delivery, connective tissue disease and other factors. Adv Clin Exp Med 21(2):207–213
Song Y, Hong X, Yu Y, Lin Y (2007) Changes of collagen type III and decorin in paraurethral connective tissue from women with stress urinary incontinence and prolapse. Int Urogynecol J Pelvic Floor Dysfunct 18(12):1459–1463. doi:10.1007/s00192-007-0356-2
Trabucco E, Soderberg M, Cobellis L, Torella M, Bystrom B, Ekman-Ordeberg G, Petraglia F, Colacurci N (2007) Role of proteoglycans in the organization of periurethral connective tissue in women with stress urinary incontinence. Maturitas 58(4):395–405. doi:10.1016/j.maturitas.2007.09.010
Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J 16(17):5353–5362. doi:10.1093/emboj/16.17.5353
Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390(6659):465–471. doi:10.1038/37284
Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P (1997) Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 389(6651):631–635. doi:10.1038/39369
Cutroneo KR, White SL, Phan SH, Ehrlich HP (2007) Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol 211(3):585–589. doi:10.1002/jcp.20972
Jimenez SA, Varga J, Olsen A, Li L, Diaz A, Herhal J, Koch J (1994) Functional analysis of human alpha 1(I) procollagen gene promoter. Differential activity in collagen-producing and -nonproducing cells and response to transforming growth factor beta 1. J Biol Chem 269(17):12684–12691
Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J (1999) Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J Invest Dermatol 112(1):49–57. doi:10.1046/j.1523-1747.1999.00477.x
Poncelet AC, Schnaper HW (2001) Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. J Biol Chem 276(10):6983–6992. doi:10.1074/jbc.M006442200
Li GY, Cui WS, Zhou F, Gao ZZ, Xin H, Liu T, Li WR, Gong YQ, Bai GY, Guo YL, Xin ZC (2012) Pathology of urethral fibromuscular system related to parturition-induced stress urinary incontinence and TGF-beta1/Smad pathway. Mol Cell Biochem 364(1–2):329–335. doi:10.1007/s11010-012-1234-x
Qiang F, Guo-long L (2011) Comparative study of three rat models of stress urinary incontinence. Bosn J Basic Med Sci 11(2):87–90
Heidkamp MC, Leong FC, Brubaker L, Russell B (1998) Pudendal denervation affects the structure and function of the striated, urethral sphincter in female rats. Int Urogynecol J Pelvic Floor Dysfunct 9(2):88–93
Pauwels E, De Wachter S, Wyndaele JJ (2009) Evaluation of different techniques to create chronic urinary incontinence in the rat. BJU Int 103(6):782–785. doi:10.1111/j.1464-410X.2008.08158.x (discussion 785–786)
Liebergall-Wischnitzer M, Paltiel O, Hochner-Celnikier D, Lavy Y, Shveiky D, Manor O (2010) Concordance between one-hour pad test and subjective assessment of stress incontinence. Urology 76(6):1364–1368. doi:10.1016/j.urology.2010.05.048
Hochstim CJ, Choi JY, Lowe D, Masood R, Rice DH (2010) Biofilm detection with hematoxylin–eosin staining. Arch Otolaryngol Head Neck Surg 136(5):453–456. doi:10.1001/archoto.2010.62
Goepel C, Thomssen C (2006) Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem 108(6):441–445. doi:10.1016/j.acthis.2006.07.001
Chen B, Yeh J (2011) Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol 186(5):1768–1772. doi:10.1016/j.juro.2011.06.054
Goepel C, Hefler L, Methfessel HD, Koelbl H (2003) Periurethral connective tissue status of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet Gynecol Scand 82(7):659–664
Edwall L, Carlstrom K, Jonasson AF (2005) Markers of collagen synthesis and degradation in urogenital tissue from women with and without stress urinary incontinence. Neurourol Urodyn 24(4):319–324. doi:10.1002/nau.20142
Wen Y, Polan ML, Chen B (2006) Do extracellular matrix protein expressions change with cyclic reproductive hormones in pelvic connective tissue from women with stress urinary incontinence? Hum Reprod 21(5):1266–1273. doi:10.1093/humrep/dei485
Wen Y, Zhao YY, Polan ML, Chen B (2008) Effect of relaxin on TGF-beta1 expression in cultured vaginal fibroblasts from women with stress urinary incontinence. Reprod Sci 15(3):312–320. doi:10.1177/1933719108315299
Zandvoort A, Postma DS, Jonker MR, Noordhoek JA, Vos JT, van der Geld YM, Timens W (2006) Altered expression of the Smad signalling pathway: implications for COPD pathogenesis. Eur Respir J 28(3):533–541. doi:10.1183/09031936.06.00078405
Medina C, Santos-Martinez MJ, Santana A, Paz-Cabrera MC, Johnston MJ, Mourelle M, Salas A, Guarner F (2011) Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol 224(4):461–472. doi:10.1002/path.2870
Moro T, Shimoyama Y, Kushida M, Hong YY, Nakao S, Higashiyama R, Sugioka Y, Inoue H, Okazaki I, Inagaki Y (2008) Glycyrrhizin and its metabolite inhibit Smad3-mediated type I collagen gene transcription and suppress experimental murine liver fibrosis. Life Sci 83(15–16):531–539. doi:10.1016/j.lfs.2008.07.023
Chiang TA, Yang YL, Yang YY, Hu MH, Wu PF, Liu SF, Huang RM, Liao TN, Hung CY, Hung TJ, Lee TC (2010) Hyperosmolarity enhanced susceptibility to renal tubular fibrosis by modulating catabolism of type I transforming growth factor-beta receptors. J Cell Biochem 109(4):663–671. doi:10.1002/jcb.22444
Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME (2006) The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J 393(Pt 2):601–607. doi:10.1042/bj20051106
Yang F, Chung AC, Huang XR, Lan HY (2009) Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension 54(4):877–884. doi:10.1161/hypertensionaha.109.136531
Acknowledgments
This study was supported by a Grant from the Science Technology and Information Bureau of Guangzhou (Grant No. 201300000137) and a fund for Excellent Talents in the Third Affiliated Hospital of Guangzhou Medical University.
Conflict of interest
We declare that we have no conflict of interest. We state that we have had full control of all primary data and we agree to allow the Journal to review our data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Liu, J., Zeng, J. et al. Expression of TβR-2, Smad3 and Smad7 in the vaginal anterior wall of postpartum rats with stress urinary incontinence. Arch Gynecol Obstet 291, 869–876 (2015). https://doi.org/10.1007/s00404-014-3495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3495-y