Abstract
Thirteen premenopausal women with stress urinary incontinence (SUI), 6 with SUI and prolapse, 9 with prolapse, and 19 without prolapse were enrolled to observe the content change of collagen type III and the expression of decorin mRNA in paraurethral connective tissues. Collagen type III from transvaginal biopsies was assayed by immunohistochemical staining and decorin mRNA was detected by real-time PCR. Premenopausal women with SUI had a significantly decreased level of collagen type III. Decorin mRNA expression was significantly increased in both premenopausal SUI+prolapse group and premenopausal prolapse group reflected by the decrease of ΔCt value compared to their corresponding controls. The results suggest that a high level of decorin mRNA might be associated with the reduced content of collagen type III, resulting in a less flexible form of extracellular matrix in the connective tissue in SUI and prolapse patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) and prolapse is a common condition with its pathogenesis not fully understood. The theory of SUI and prolapse, presented by Petros and Ulmsten and Liu et al. [1, 2], put particular emphasis on the characteristics of the connective tissue in the female pelvic floor. As collagen is important in determining the elastic behavior of the connective tissue in the pelvic floor, many researchers have focused on the change of collagen in ligaments and fasciae mainly consisting of the connective tissue. Decorin, a small leucine-rich proteoglycan that regulates collagen fibrillogenesis, might play an important role in histomorphogenesis. We hypothesize that both collagen and decorin will be altered in SUI and prolapse and are possibly associated with these conditions. The current work was aimed to see whether the quantity of collagen type III and the expression of decorin mRNA were changed in paraurethral connective tissue from premenopausal women with SUI and women with SUI and prolapse.
Materials and methods
A total of 47 patients participated in the study. For collagen assay, 25 premenopausal women who were hospitalized from August 2003 to December 2004 were enrolled. Among them, 13 subjects with SUI were pooled together as the SUI group and the other 12 without SUI as the control group. SUI was defined by gynecologic examination, pad test, and urodynamic examination according to the International Continent Society standard [3]. The control group consisted of premenopausal women who underwent hysterectomy for benign conditions. For decorin mRNA test, 22 patients hospitalized from June 2006 to November 2006 were divided into SUI+prolapse group (6 patients: 4 premenopausal and 2 postmenopausal), prolapse group (9 patients: 3 premenopausal and 6 postmenopausal), and control group (7 premenopausal patients with benign uterine muscular tumors). Patients with previous operation for urinary incontinence or anterior vaginal prolapse were excluded from the study. Patients who had used estrogen in the previous 3 months were not included either. All the women in the control group were excluded from SUI or prolapse. The study was approved by the Hospital Scientific Committee. All patients consented to participate in the clinical trial and agreed to have their biopsies performed.
Full-thickness samples, measured roughly 5 mm in diameter, were removed from the anterior vaginal wall at the level of the bladder neck during the surgical procedure. For collagen assay, the sample was fixed in 10% paraformaldehyde for immunohistologic analysis. For decorin mRNA test, the tissue was immediately stored in RNAlater (Qiagen, Hilden, Germany) for total RNA isolation.
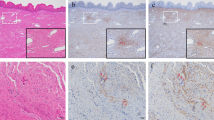
The presence of collagen type III was determined by immunohistochemical staining. The steps were performed using the Immuno-stain EnVision kit (Biogenex, San Ramon, CA, USA) according to the manufacturer’s instructions. Briefly, the sections from each sample were stained immunohistochemically with mouse anti-human collagen type III antibody followed by polyperoxidase-linked IgG (Zymed, San Francisco, CA, USA) for 30 min. AEC (3-amino-9-ethylcarbozole) was used as a chromogen to react with peroxidase for 5 min. Negative controls were produced by replacing the primary antibody with Tris buffer. All biopsies were performed by one surgeon and immunohistochemical analysis was performed by a histopathologist who was blinded to the experimental arrangement. A weak or no staining reaction was marked as (−), a moderate staining reaction marked as (+), and a strong staining reaction marked as (++) [4]. The data were analyzed and compared in relation to the collagen type III content in the two groups. The chi-square test was used for statistical analysis and a p value <0.05 was considered statistically significant.
Copy numbers of decorin mRNA in the anterior vaginal wall were estimated by the Ct method of relative quantification using an optimized real-time quantitative PCR with SYBR Green I fluorescent detection. The sample was homogenized in Trizol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the instructions of the kit. The total RNA was dissolved in 50 μl of RNase-free water with its concentration measured by a UV spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan). The primers were designed by Shanghai Shenggong Biology using the online software Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Their sequences were: decorin 5′-gga ccgtttcaacagagagg-3′ (forward) and 5′-gagttgtgtcagggg gaaga-3′ (reverse), which amplified a 205 bp product; β-actin 5′-agagctacgagctgcctgac-3′ (forward), 5′-agcac tgtgttggcgtacag-3′ (reverse), which amplified a 184 bp product. Reverse transcription was performed from 1 μg of total RNA using oligodT primers according to the manufacturer’s instructions (Promega). Real-time PCR was performed to quantify the samples’ cDNA copies using SYBR premix ExTaqTM fluorescent quantitation PCR kit (SYBR Premix ExTaqTM, TaKaRa, Japan) in the iCycler iQ real-time PCR device (BioRad, Germany) after a conventional PCR to optimize the amplification condition in a PCR thermal cycler (PE2400, Perkin-Elmer, USA). The total 12.5 μl reaction system that was placed in a 96-well plate comprised of deionized water 5 μl, forward and reverse primers mixture 0.25 μl, SYBR mixture 6.25 μl, and cDNA sample 1 μl. The thermocycler program was set as 94°C 40 s, 57°C 45 s, 72°C 45 s for 45 cycles, paralleled to the same program for β-actin’s reaction system. The number of cDNA copies were doubly quantified for decorin and β-actin genes. The average value was used as the result. At the end of each reaction, the cycle threshold (Ct) was manually set up at the level that reflected the best kinetic PCR parameters, and melting curves were acquired and analyzed.
In the present work, the 2−ΔΔCt method of relative quantification was adapted to estimate the copy numbers in the decorin gene. This method allows to estimate gene copy number relativity in unknown samples. For each reaction, relative mRNA copies were expressed as the relative expression rate 2−ΔΔCt where ΔΔCt=ΔCt (observed)−ΔCt (controlled) and ΔCt=Ct (decorin)−Ct (β-actin). Ct stands for the number of cycles when the fluorescent signal reached the upper threshold in each reaction tube. There is a negative linear relationship between Ct and the logarithm of initiating copies in a certain template. ΔCt was applied as the result to calculate the differences between observed and control groups. Data were presented as the mean±standard deviation (SD). Differences of the mean values between the two groups were analyzed by the unpaired Student’s t test. Differences of the mean values among multi-groups were analyzed using the one-way ANOVA followed by pair-wise comparisons with Newman–Keuls post hoc testing when the ANOVA showed significant differences. A p value <0.05 was considered statistically significant.
Results
The patients in the collagen observation groups were in the proliferative phase of the menstrual cycle, as verified by uterine histological evidence. There was no significant difference in age, parity, and body mass index (BMI) between the SUI group and the control group (p > 0.05). The patients in the decorin mRNA groups showed no significant difference in the parameters, except for the age between prolapse and control groups. No significant difference for age was found between SUI+prolapse group and control group, as listed in Table 1.
Collagen type III immunohistochemical staining reaction in paraurethral connective tissue in premenopausal patients with and without SUI are shown in Fig. 1 and Table 2. As seen from Table 2, there was a significantly lower constituent ratio of staining reaction for (++) in the SUI group than in the control group (p < 0.05).
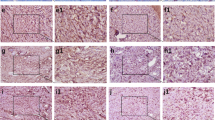
The expression of decorin mRNA in paraurethral connective tissue are shown in Figs. 2 and 3. Compared to their corresponding controls, ΔCt for decorin mRNA expression was significantly reduced in both premenopausal SUI+prolapse group (p < 0.05, Fig. 2) and premenopausal prolapse group (p < 0.01, Fig. 3). When comparison was made between SUI+prolapse or prolapse group (consisting of both premenopausal and postmenopausal patients) and their control groups, ΔCt for decorin mRNA expression was decreased without statistically significant difference. Compared to the control group using the mean 2−ΔΔCt as the result, the expression of decorin mRNA was significantly elevated by 2.07 times (2−ΔΔCt = 2.074, premenopausal SUI+prolapse vs control) in the premenopausal SUI+prolapse group and by 2.11 times (2−ΔΔCt = 2.107, premenopausal prolapse vs control) in the premenopausal prolapse group. No significant difference was found between SUI+prolapse and prolapse groups where the 2−ΔΔCt values were 1.850 and 0.965, respectively.
Discussion
SUI is a common condition and a major cause of gynecological surgery. Nearly 12.5% of women suffer from different degrees of SUI [5]. Recent research suggests that SUI is due to the inability of the urethral sphincter mechanics to maintain a higher pressure in the urethra than in the bladder during stress. Prolapse refers to the departure of the anatomically normal position of the pelvic organs due to the defect or chalasis of the pelvic supporting tissue, including the prolapse of the genital tract, bladder, rectum or small intestine that ambulates into the vagina. Pelvic relaxation, a weakening of the pelvic support structures, is a condition that results in prolapse. Decreased collagen content has been noted in the tissues of women affected by this condition [6]. The connective tissue of the pelvic floor plays an important role in maintaining the continent mechanism by providing an additional occlusive force on the urethral wall, particularly when intra-abdominal pressure increases. Extracellular matrix of the connective tissue is comprised of collagen and elastin fibers, glycoproteins, and proteoglycans, among which collagen fibers are the main component. A series of studies have found a significant reduction in the collagen content in the endopelvic fascia of patients with SUI [4, 7–9]. A reduced quantity of collagen in the connective tissue leads to a poor quality of fascia with reduced tensile strength; therefore, impairs the functional relation between fascia and muscles and causes weakness of the muscular support. Nineteen genetically distinct types of collagen have been identified so far with collagen type I and type III being the major structural components. Collagen type III is considered to contribute more to the elastic property of the tissue, which is important in maintaining both the position of the organs and the pressure of the urinary tract [4, 10, 11].
Decorin, an extensively distributed small leucine-rich proteoglycan binding to collagen fibrils via its core protein to regulate collagenesis in vitro and in vivo, may play an important role in the mechanics of the connective tissue [12]. In vitro experiments demonstrated that a targeted disruption of the decorin gene produces collagen fibrils that are irregular in size and in diameter, suggesting an impaired cross-linking [13]. In another study, decorin was found to reduce the production of collagen type III by about 80% in Tenon’s capsule [14]. Therefore, it is reasonable to postulate that decorin is likely to impact the functional property of collagen-dependent tissue such as elasticity.
In the present study, we observed the content change of collagen type III and the expression of decorin mRNA in paraurethral connective tissue from premenopausal women with SUI and women with SUI+prolapse, respectively. The content of collagen type III was significantly lower in patients with SUI than that in patients without SUI, which is consistent with the data previously reported [4]. It is possible that the reduction of collagen type III is one of the main factors contributing to the development of SUI and prolapse. In the present work, we also found a significantly reduced ΔCt for decorin mRNA expression in both premenopausal SUI+prolapse group and premenopausal prolapse group, reflecting a significant increase in decorin mRNA expression. When using the mean 2−ΔΔCt as the result, the expression of decorin mRNA was markedly elevated by 2.07 times (2−ΔΔCt = 2.074) in the premenopausal SUI+prolapse group and by 2.10 times (2−ΔΔCt = 2.107) in the premenopausal prolapse group compared to their control groups, respectively, giving a relative change level between the observed group and the control group. Although there was a tendency of ascent in decorin mRNA expression when compared between SUI+prolapse or prolapse group (consisting of both premenopausal and postmenopausal patients) and their control groups, the discrepancy of decreased ΔCt for decorin mRNA expression to the control did not reach a statistically significant difference. The possible explanation for this might be that decorin mRNA expression in postmenopausal patients was compromised after their menopause, as there was no significant difference of decorin mRNA expression between the postmenopausal group and control group, suggesting a reduced decorin mRNA expression. Low levels of estrogen after menopause might be associated with the decreased decorin mRNA expression in these patients. A larger sample is needed for further observation. Another problem was the difficulty of collecting postmenopausal control patients. All the patients in the control group were premenopausal, which affected the comparability between observed and control groups. No significant difference was found between SUI+prolapse and prolapse groups where the 2−ΔΔCt values were 1.85 and 0.965, respectively.
A series of observations provide evidences that decorin might not only retard collagen fibril formation but also influence its diameter [15, 16]. The expression of decorin DNA was increased in premenopausal women with SUI in comparison with their continent counterpart [17]. Our data of decorin mRNA were consistent with these previous findings. Besides, our data showed that the expression of decorin mRNA was also increased in either SUI+prolapse patients or prolapse patients, which suggests that there might be a partially common mechanism shared for the pathogenesis of both SUI and prolapse processes. Collagen type III contributes to the physical property of the tissue and is involved in its resistance and elasticity. As decorin is proved to significantly reduce the content of collagen type III, these alterations should result in a less flexible form of extracellular matrix in paraurethral connective tissue. Taken together, it is likely that the increased expression of decorin mRNA in the pelvic floor tissue is closely related to the susceptibility and development of both SUI and prolapse.
References
Petros PE, Ulmsten UI (1990) An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl 153:7–31
Liu X, Zhao Y, Pawlyk B, Damaser M, Li T (2006) Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol 168:519–528
Abrams P, Blaivas JG, Stanton S, Andersen JT (1988) The standardisation of terminology of lower urinary tract function. Neurourol Urodyn 7:403–426
Liapis A, Bakas P, Pafiti A, Frangos-Plemenos M, Arnoyannaki N, Creatsas G (2001) Changes of collagen type III in female patients with genuine stress incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod Biol 97:76–79
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S (2000) A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord Trondelag. J Clin Epidemiol 53:1150–1157
Salter SA, Batra RS, Rohrer TE, Kohli N, Kimball AB (2006) Striae and pelvic relaxation: two disorders of connective tissue with a strong association. J Invest Dermatol 126(8):1745–1748
Rechberger T, Postawski K, Jakowicki JA, Gunja-Smith Z, Woessner JF Jr (1998) Role of fascial collagen in stress urinary incontinence. Am J Obstet Gynecol 179:1511–1514
Keane DP, Sims TJ, Abrams P, Bailey AJ (1997) Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol 104:994–998
Bergman A, Elia G, Cheung D, Perelman N, Nimni ME (1994) Biochemical composition of collagen in continent and stress urinary incontinent women. Gynecol Obstet Invest 37:48–51
Nimni ME (1983) Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum 13:1–86
Weber KT (1989) Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13:1637–1652
Vesentini S, Redaelli A, Montevecchi FM (2005) Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J Biomech 38:433–443
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136:729–743
Mietz H, Chevez-Barrios P, Lieberman MW, Wendt M, Gross R, Basinger SF (1997) Decorin and suramin inhibit ocular fibroblast collagen production. Graefes Arch Clin Exp Ophthalmol 235:399–403
Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR (2000) Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci 57:859–863
Watanabe T, Hosaka Y, Yamamoto E, Ueda H, Sugawara K, Takahashi H, Takehana K (2005) Control of the collagen fibril diameter in the equine superficial digital flexor tendon in horses by decorin. J Vet Med Sci 67:855–860
Chen B, Wen Y, Zhang Z, Wang H, Warrington JA, Polan ML (2003) Menstrual phase-dependent gene expression differences in periurethral vaginal tissue from women with stress incontinence. Am J Obstet Gynecol 189:89–97
Acknowledgements
The authors gratefully acknowledge Mr. Shuiliang Wang and Mrs. Liqing Yao for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a grant from the Fujian Science and Technology Bureau Foundation (grant no. 2000I1003).
Rights and permissions
About this article
Cite this article
Song, Y., Hong, X., Yu, Y. et al. Changes of collagen type III and decorin in paraurethral connective tissue from women with stress urinary incontinence and prolapse. Int Urogynecol J 18, 1459–1463 (2007). https://doi.org/10.1007/s00192-007-0356-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-007-0356-2