Purpose
To study the consistency of hysteroscopy findings and histological chronic endometritis (CE) in recurrent implantation failure (RIF) cases, and to compare their values in indicating antibiotic treatment.
Methods
Sixty RIF cases (January 2009–January 2010) and 202 consecutive RIF cases (May 2010–April 2012) in Peking University Third Hospital reproductive medical center were studied. 60 RIF patients’ endometrial samples redid section and CD38/CD138 immunohistochemical stain for CE screening. In 202 RIF cases, the presence of hyperemia, mucosal edema, and micropolyps under hysteroscopy were considered CE diagnostic parameters. Antibiotic was offered to part of the patients. The patients’ clinical outcomes were analyzed by statistical methods.
Results
In 202 RIF cases, the hysteroscopy CE rate was 66.3 %, while histological CE rate was 43.6 %. The sensitivity and specificity of hysteroscopy were 35.2 and 67.5 %. In histological CE patients, 68 cases underwent regular antibiotic treatment and 20 did not. Two groups had similar clinical pregnancy rates (35.3 vs. 30.0 %), embryo implantation rates (18.9 vs. 20.4 %) and ongoing pregnancy rates (29.4 vs. 25.0 %). In hysteroscopy CE patients, the implantation rate (18.6 vs. 4.9 %) and ongoing pregnancy rate (29.3 vs. 7.4 %) significantly increased (P < 0.05) with antibiotic treatment, and higher intrauterine pregnancy rate in treatment group (29.3 vs. 11.1 %). In reviewing the chosen 60 RIF cases, the histological CE rates were similar in both pregnancy and non-pregnancy group after subsequent embryo transfer.
Conclusions
CE occurs frequently in RIF patients; hysteroscopy has more diagnostic and treatment value for them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A functioning and receptive endometrium is crucial for embryo implantation. During women’s menstrual cycle, the endometrium undergoes both morphologic and biologic changes, preparing for interaction with the embryo, which leads to successful implantation. Once all biological changes are adequate, the embryo can attach and invade the endometrium, and finally implant.
Chronic endometritis (CE) is a persistent inflammation of the endometrial lining. Histologically, the diagnosis of CE is generally based on the presence of an excessive number of plasma cells infiltrating in endometrial biopsies [1, 2]. CE is often asymptomatic or accompanied only by mild symptoms. Although it is often clinically silent, CE may hamper the reproductive capacity of both spontaneous and in vitro fertilization (IVF) cycles [3]. Moreover, compelling evidence shows that CE may also cause spontaneous preterm labor [4]. It is reported that the prevalence of CE in recurrent implantation failure (RIF) patients of IVF cycles is significantly higher [5].
For RIF cases, office hysteroscopy can perform diagnosis and treatment [6]. CE is often diagnosed by the combination of hysteroscopy and endometrial biopsy [7, 8]. However, different endometrial biopsy methods and different methods of making and reading pathological section often lead to a great fluctuation of CE diagnostic rate [3, 9, 10]. The consistency between abnormal findings through hysteroscopy and histological CE needs to be further examined. Whether embryo implantation rate can be increased with full-dose full-period use of antibiotics also needs to be confirmed for RIF patients with CE.
In this study, we did hysteroscopy and endometrial biopsy for RIF cases in IVF, in order to examine the consistency between abnormal hysteroscopy finding and histological CE. We also investigated the significance of antibiotics use for RIF patients who have abnormal hysteroscopy results or histological CE.
Materials and methods
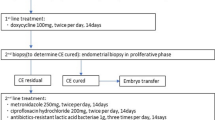
Part 1: 202 consecutive RIF cases in Peking University Third Hospital reproductive center from May 2010 to April 2012 were studied. Exclusion criteria were uterine abnormality (septum, unicornuate uterus), submucous myoma, adhesion of uterine cavity, and endometrial hyperplasia or tuberculosis by histological diagnosis.
Part 2: 60 RIF cases from January 2009 to January 2010 who did not receive hysteroscopy or antibiotic treatment, but only endometrial biopsy were studied. Half of these 60 cases got pregnant in subsequent cycle, and the other half did not.
RIF is defined as the failure to implant after three IVF treatment attempts or after ≥6 high-quality embryo transfers [11]. Good-quality embryo is defined as embryo reaching blastocyst stage or at least six cells on day 3 with grading of one or two. All patients received non-ultrasound-guided embryo transfer. The endometrial biopsy was conducted by Pipelle aspiration.
In part 1 cases, all women underwent diagnostic office hysteroscopy in the follicular phase of the menstrual cycle by the same two doctors (Dr. Yang and Dr. Song).
The hysteroscopies were performed on an outpatient basis with the use of a 5-mm (outer diameter) continuous-flow hysteroscopy (Olympus). The endocervical canal, uterine cavity, tubal orifices, and endometrium were inspected methodically and the findings were recorded.
The exploration of the uterine cavity consisted of a panoramic view of the cavity followed by a thorough evaluation of the endometrial presence of hyperemia (see Fig. 1), mucosal edema, and micropolyps (see Figs. 2, 3) as diagnostic parameters.
Antibiotic treatment was offered to patients with histological chronic endometritis (2 weeks of levofloxacin 0.5 g qd and metronidazole 1 g qd); some patients refused this treatment.
In part 2, we redid the pathological section and hematoxylin-eosin staining as well as CD38 and CD138 immunohistochemical staining on endometrial biopsy samples of the 60 RIF patients (see Figs. 4, 5).
The research protocol was approved by the Ethics Committee of Peking University.
The patients’ age, infertile duration, numbers of embryos transferred, and clinical outcomes were compared. Measurements are presented as mean ± SD. For statistical analysis, we used the Statistical Package for Social Sciences (SPSS, version 16.0, SPSS Inc, Chicago, IL) for Windows. The Student’s t test and Chi-squared (X 2) test were used to compare the categorical variables. A P value <0.05 was considered statistically significant.
Results
-
1.
Sensitivity and specificity of hysteroscopy in diagnosis of histological CE.
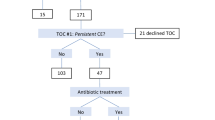
In 202 consecutive RIF cases in part 1, the uterine cavity abnormality, such as mucous hyperemia, edema, and micropolyps, was found in 66.3 %, while histological CE was in 43.6 % of all RIF patients. Compared with histological result, the sensitivity and specificity of hysteroscopy were 35.2 and 67.5 %. The false-positive rate was 64.8 %, and false-negative rate is 22.5 % (see Table 1).
-
2.
Antibiotic treatment effects in histological CE
We compared histological CE patients who underwent regular antibiotic treatment (68) and those who did not (20). 24 cases in the treatment group got pregnant after embryo transfer (ET) (35.3 %), the remaining 44 cases had implantation failure. In the non-treatment group, 6 cases got pregnant after ET (30 %), the remaining 14 cases had implantation failure. The embryo implantation rates (18.9 vs. 20.4 %) and ongoing pregnancy rates (29.4 vs. 25.0 %) of the two groups are similar. Only the previous numbers of implantations in the non-treatment group are higher than the treatment group (see Table 2).
-
3.
Antibiotic treatment effects in hysteroscopy CE
With uterine cavity abnormality such as mucous hyperemia, edema, and micropolyps, no matter whether histological CE was diagnosed or not, the implantation rate and ongoing pregnancy rate increased significantly (P < 0.05) with antibiotic treatment. The intrauterine pregnancy rate was also higher in treatment group, but there was no statistical difference (see Table 3).
-
4.
Retrospective analysis of histological and clinical results of 60 RIF cases
To further determine whether histological CE diagnosis would affect clinical outcomes after IVF-ET, we selected 60 RIF and reviewed their endometrial samples.
The results showed that 9 in 30 patients who got pregnant in subsequent ET cycle had histological CE (30 %), and 13 in 30 who did not get pregnant had histological CE (43.3 %). The age, infertile duration, failure times and number of good-quality embryo transfer were similar in the two groups (see Table 4).
Discussion
Implantation is a highly complex process which involves two aspects: mother and embryo [12]. The maternal environment abnormality such as anatomic abnormality, low endometrial receptivity, maternal thrombolic and immunologic response abnormality will negatively affect the crosstalk between maternal body and embryo [13]. Immunologic response involves a physiologic inflammatory process characterized by leucocytes, immunoglobulins, cytokines, and other factors in the endometrium. These cells and their inflammatory mediators are important in the regulation of the immunoresponse and trophoblast growth [5].
Neelam [14] reported a retrospective meta-analysis in 2001, and CE was thought to be an important factor which caused RIF. CE is often asymptomatic or accompanied only by mild symptoms, which include pelvic pain, dysfunctional uterine bleeding, dyspareunia, and leucorrhea. Due to its subtle nature, the actual prevalence of this pathology in the general population is unknown. Romero [5] found 15 % of infertile women had CE, while in RIF patients, the occurrence rate increased to 42 %. In 2010, Erike reported 30.3 % of histological CE were found in RIF patients screening [10]. The highest histological CE rate in RIF cases was 60 %, reported by Conway in 2010 [15].
Many studies found that hysteroscopy is a meaningful test for RIF cases following IVF procedure [6–8, 16]. A prospective study by Polisseni et al. [9] did diagnostic hysteroscopy, endometrial biopsy, and cervical and endometrial Chlamydia infection test on 50 infertile patients. Hematoxylin–eosin staining proved that CE occurred in 12 % of these patients and had no relation to Chlamydia infection. Hysteroscopy had a sensitivity of 16.7 % and specificity of 93.2 % in diagnosing histological CE, and was considered to have a high predictive value of negative result. Cicinelli et al. [17] considered polyps smaller than 1 mm in diameter to be “micro polyps”, and were often associated with histological CE, with a correlation higher than 90 % between hysteroscopy and pathological findings. Svirsky [18] reported in 2008 that in 639 cases, hysteroscopy combined with endometrial biopsy had a much higher diagnostic rate in detecting abnormal uterine cavity than biopsy alone. Endometrial biopsy had a false-negative rate of 88.7 % in diagnosing endometrial polyps, and hysteroscopy could not make a definite diagnosis of hyperplasia.
In our data for RIF patients, both hysteroscopy and histology CE abnormality rate were high. Under hysteroscopy, mucous hyperemia, edema, and micropolyps were found in 66.3 % cases, while histological CE was found in 43.6 % cases. Hysteroscopy diagnosis for histological CE had a 35.2 % of sensitivity, and 67.5 % of specificity, which also showed a higher negative predictive value.
Although infiltration of the endometrium by lymphocytes is associated with chronic endometritis, the diagnosis is ultimately based on the presence of plasma cells in the endometrial stroma. The search for plasma cells may be hampered by such factors as inadequate staining, preservation of the endometrial tissue, or mimicking of plasma cells by plasmacytoid stroma cells. All these make the search for plasma cells very difficult, which in turn makes the pathological diagnosis of chronic endometritis difficult [19]. Currently, immunohistochemical staining is recommended on endometrial specimen to test plasma cell-specific surface antigens CD38 and CD138, in order to confirm the presence of plasma cells, although sometimes the above factors are still inevitable [20]. Also, since Pipelle tube is used to draw materials for endometrial biopsy, a non-specific method, the specimen gathered may not be the abnormal endometrial tissue shown by the hysteroscopy, which is a limitation in the pathological diagnosis of CE. This may account for the discrepancy between the CE diagnostic value of hysteroscopy and histological observation.
Most studies showed infection is the basis of CE. Cicinelli [21] reported a study in 2008 in which 2,190 cases were included, and they all received hysteroscopy and endometrial biopsy. Pathogen test of endometrium and vagina were done in 438 hysteroscopic CE cases, and 100 non-CE cases as control. Culture of common bacterial, Neisseria gonorrhea and Mycoplasma, and molecular biology testing for Chlamydia were performed. The result showed 388 hysteroscopic CE cases (88.6 %) turned out to be histological CE, and 320 (73.1 %) of them had at least one kind of pathogen positive. In the control group, 6 % cases found histological CE, and culture-positive rate was only 5 %. Most endometrial infections originated from common bacterial infection (58 %), and Ureaplasma urealyticum was detected in 10 % and Chlamydia in only 2.7 % of positive endometrial cultures. They concluded that common bacteria and Mycoplasma were the most frequent etiologic agents.
Gram-negative bacterial colonization in endometrium can interfere with the regulation process, thereby lowering the implantation rate while increasing abortion rate [22]. Substantial evidence links the failure of successful pregnancy to the cytokine profile produced by two subgroups of T cells. Endotoxins, components of the cell wall of the gram-negative bacteria, stimulate the production of proinflammatory cytokines with a more predominant TH1 response in the decidua (such as macrophages tumor necrosis factor-α and interleukin-1β) that may predispose the environment toward damage to the embryo, implantation failure, or spontaneous abortion.
For histologically confirmed CE patients, the use of full-dose antibiotics is the currently acknowledged treatment method. We treated CE patients with 2 weeks of levofloxacin 0.5 g qd and metronidazole 1 g qd. But the undesirable influence of antibiotic treatment of CE on IVF outcomes for RIF patients are reported as follows: Lamonica et al. [23] studied 26 cases which had at least two failed IVF-ET and diagnosed CE through cell-specific antigen CD138 staining. The results showed that even when endometrium biopsy findings turned normal after antibiotics treatment, the implantation rate and clinical pregnancy rate were low in the subsequent transfer cycle. Andrews et al. [24] reported a prospective study which showed that antibiotic treatment for CE patients does not lower the occurrence of abortion and premature delivery. Erika et al. [10] reported 33 RIF cases with CE which, after antibiotic treatment, still had lower implantation rate in the subsequent transfer cycle than the control group, but with similar clinical and ongoing pregnancy rates. Despite these reports that antibiotics can cure pathological CE (absence of plasma cell in endometrium under microscopy), all the studies showed antibiotics cannot improve the ultimate clinical IVF outcomes.
Some other studies reported that pathological CE, even untreated, does not affect clinical IVF outcomes. Fatemi [25] published a case report in 2009, a CD138-positive, diagnosed chronic endometritis and refused antibiotic treatment patient, got pregnant and had term delivery in the first attempt of IVF. The author believed CE may not lower IVF success rate. In the paper of a randomized controlled trial by Kasius et al. [26], a hysteroscopy-guided endometrial biopsy was obtained and histologically examined in 678 infertile women. The live birth rate after initiation of IVF/ICSI treatment of patients diagnosed with chronic endometritis was compared with a randomly selected matched control group of patients without endometritis. The prevalence of histological CE was 2.8 %. The cumulative live birth rate (76 vs. 54 %) and clinical pregnancy rate per embryo transfer (hazard ratio 1.456, 95 % CI 0.770–2.750) did not significantly vary between patients with or without endometritis. They concluded the reproductive outcome after initiation of IVF/ICSI was not found to be negatively affected by histological CE.
Our study also shows in histological CE cases, embryo implantation rates, clinical pregnancy rates and ongoing pregnancy rates are similar between groups with and without antibiotic treatment. In reviewing selected 60 RIF patients in Part 2, we found that between pregnancy group and non-pregnancy group, the histological CE diagnosis rates were similar. Therefore, we believe endometrial biopsy cannot indicate the overall uterine cavity and endometrial states, since it is based on limited sample collection. On the other hand, embryo implantation may not demand such perfect condition as we thought. Even pathologically and immunohistochemically confirmed CE may not have great effect on implantation. Clinical RIF may have more complex and invisible causes for us to investigate [27]. Therefore, we believe Pipelle biopsy has only limited clinical value to diagnose CE, and may not be suitable for extensive screening for RIF patients.
Some other studies [3] suggest that untreated CE is detrimental to the IVF outcomes. However, the CE mentioned in these articles was diagnosed under hysteroscopy examination. Our study found that for RIF patients who were diagnosed CE due to abnormal hysteroscopy findings, antibiotic treatment can greatly increase implantation rate (18.6 vs. 4.9 %) and ongoing pregnancy rate (29.3 vs. 7.4 %) in subsequent transfer cycle (P < 0.05). Hysteroscopy can cover all uterine cavity, which facilitates the observation of endometrial thickness and vessel-filling state so that endometrial receptivity can be evaluated. CE diagnosed through hysteroscopy is likely to be correlated with chronic infection, since antibiotic treatment can clean the occult bacterial infection and increases embryo implantation rate.
To sum up, RIF is a difficult problem in IVF-ET, and the causes need to be investigated. CE occurs frequently in RIF patients, but the diagnosis methods are imperfect. Our results showed the discrepancy between pathology and hysteroscopy. In the clinical practice, diagnostic hysteroscopy should be a regular test for RIF patients, exploring the whole uterine cavity and detecting the presence of hyperemia, mucosal edema, and micropolyps, manifestations that can easily be ignored. The diagnosis of histological CE may be hampered by inadequate staining and preservation of the endometrial tissue, or plasma cell mimicking; Pipelle aspiration cannot show the whole endometrium condition, and so the antibiotic treatment cannot enhance IVF outcomes, though it can, sometimes, improve pathological manifestations. Therefore, we may conclude that diagnostic hysteroscopy has more clinical value in diagnosing infectious CE and the further antibiotic treatment for RIF patients.
This is a retrospective study with limited data. It requires prospective random controlled trial to further determine the mechanism of how histological CE and hysteroscopy CE influence the subsequent cycle IVF outcome of RIF patients.
References
Kiviat NB, Wolner-Hanssen P, Eschenbach DA et al (1990) Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol 14:167–175
Cravello L, Porcu G, D’Ercole C et al (1997) Identification and treatment of endometritis. Contracept Fertil Sex 25:585–586
Feghali J, Bakar J, Mayenga JM et al (2003) Systematic hysteroscopy prior to in vitro fertilization. Gynecol Obstet Fertil 31:127–131
Espinoza J, Erez O, Romero R (2006) Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol 194:630–637
Romero R, Espinoza J, Mazor M (2004) Can endometrial infection/inflammation explain implantation failure, spontaneous abortion and preterm birth after in vitro fertilization? Fertil Steril 82:799–804
Ercan CM, Ozturk M, Dede M et al (2011) Narrow band imaging hysteroscopy: a new diagnostic technique in recurrent IVF failure? Arch Gynecol Obstet 283(Suppl 1):135–136
Pedro A, Jorge M, Fonseca E et al (2004) What is the role of routine hysteroscopy in the evaluation of uterine cavity in infertile women? Int Congr Ser 1271:259–262
Oliveira FG, Abdelmassih VG, Diamond MP et al (2003) Uterine cavity findings and hysteroscopic interventions in patients undergoing in vitro fertilization-embryo transfer who repeatedly cannot conceive. Fertil Steril 80(6):1371–1375
Polisseni F, Bambirra EA, Camargos AF (2003) Detection of chronic endometritis by diagnostic hysteroscopy in asymptomatic infertile patients. Gynecol Obstet Invest 55:205–210
Johnston-MacAnanny EB, Hartnett J, Engmann LL et al (2010) Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril 93(2):437–441
Rinehart J (2007) Recurrent implantation failure: definition. J Assist Reprod Genet 24:284–287
Simon A, Laufer N (2012) Repeated implantation failure: clinical approach. Fertil Steril 97(5):1039–1043
Margalioth EJ, Ben-Chetrit A, Gal M et al (2006) Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod 21:3036–3043
Neelam P, Vitthala S (2001) Are the causes of recurrent implantation failure a myth evidence based reality? Fertil Steril S279:P-582
Conway DA, Ketefian A, Shamonki M (2010) Chronic endometritis: A common finding in good prognosis patients with failed implantation following IVF. Fertil Steril 94(4):S175
Makled AK, Farghali MM, Shenouda DS (2014) Role of hysteroscopy and endometrial biopsy in women with unexplained infertility. Arch Gynecol Obstet 289(1):187–192
Cicinelli E, Resta L, Nicoletti R et al (2005) Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod 20:1386–1389
Svirsky R, Smorgick N, Rozowski U et al (2008) Can we rely on blind endometrial biopsy for detection of focal intrauterine pathology? Am J Obstet Gynecol 199:115.e1–115.e3
Adegboyega PA, Pei Y, McLarty J (2010) Relationship between eosinophils and chronic endometritis. Hum Pathol 41:33–37
Bayer-Garner IB, Nickell JA, Korourian S (2004) Routine syndecanl immunohistochemistry aids in the diagnosis of endometritis. Arch Pathol Lab Med 128:1000–1003
Cicinelli E, De Ziegler D, Nicoletti R et al (2008) Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril 89:677–684
Kamiyama S, Teruya Y, Nohara M et al (2004) Bacterial endotoxin in the endometrium and its clinical significance in reproduction. Fertil Steril 82:805
Lamonica R, Hartnett J, Engmann LR et al (2006) Immunohistochemistry confirms the presence of chronic endometritis in patients with recurrent implantation failure. Fertil Steril 86:S280
Andrews WW, Goldenberg RL, Hauth JC et al (2006) Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstet Gynecol 194:617–623
Fatemi HM, Popovic-Todorovic B, Ameryckx L et al (2009) In vitro fertilization pregnancy in a patient with proven chronic endometritis. Fertil Steril 91(4):1293.e9–1293.e11
Kasius JC, Fatemi HM, Bourgain C et al (2011) The impact of chronic endometritis on reproductive outcome. Fertil Steril 96:1451–1456
Punnonen R, Lehtinen M, Teisala K et al (1989) The relation between serum sex steroid levels and plasma cell infiltrates in endometritis. Arch Gynecol Obstet 244(4):185–191
Acknowledgments
We appreciate Dr. Congrong Liu and Qian Yao from Department of Pathology of Peking University Third Hospital for providing pathological support, and Dr. Hai Na for his assistance in revision of the article.
Conflict of interest
There are no conflicts of interest for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Yang and X. Du were joint first authors of this article.
Rights and permissions
About this article
Cite this article
Yang, R., Du, X., Wang, Y. et al. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstet 289, 1363–1369 (2014). https://doi.org/10.1007/s00404-013-3131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-3131-2