Abstract
Purpose
To investigate the impact of antibiotic treatment for chronic endometritis (CE) on the pregnancy outcome of frozen-thawed embryo transfer (FET) cycles and the relevant clinical risk factors associated with CE.
Methods
A retrospective cohort analysis was conducted on 1352 patients who underwent hysteroscopy and diagnostic curettage at Nanjing Maternal and Child Health Hospital from July 2020 to December 2021. All patients underwent CD138 immunohistochemical (IHC) testing to diagnose CE, and a subset of them underwent FET after hysteroscopy. Patient histories were collected, and reproductive prognosis was followed up.
Results
Out of 1088 patients, 443 (40.7%) were diagnosed with CE. Univariate and multivariate binary logistic regression analyses revealed that parity ≥ 2, a history of ectopic pregnancy, moderate-to-severe dysmenorrhea, hydrosalpinx, endometrial polyps, a history of ≥ 2 uterine operations, and RIF were significantly associated with an elevated risk of CE (P < 0.05). Analysis of the effect of CE on pregnancy outcomes in FET cycles after antibiotic treatment indicated that treated CE patients exhibited a significantly lower miscarriage rate (8.7%) and early miscarriage rate (2.9%) than untreated non-CE patients (20.2%, 16.8%). Moreover, the singleton live birth rate (45.5%) was significantly higher in treated CE patients than in untreated non-CE patients (32.7%). Survival analysis revealed a statistically significant difference in the first clinical pregnancy time between treated CE and untreated non-CE patients after hysteroscopy (P = 0.0019). Stratified analysis based on the presence of recurrent implantation failure (RIF) demonstrated that in the RIF group, treated CE patients were more likely to achieve clinical pregnancy than untreated non-CE patients (P = 0.0021). Among hysteroscopy-positive patients, no significant difference was noted in pregnancy outcomes between the treatment and control groups (P > 0.05).
Conclusion
Infertile patients with a history of parity ≥ 2, hydrosalpinx, a history of ectopic pregnancy, moderate-to-severe dysmenorrhea, endometrial polyps, a history of ≥ 2 uterine operations, and RIF are at an increased risk of CE; these patients should be recommended to undergo hysteroscopy combined with CD138 examination before embryo transfer. Antibiotic treatment can improve the reproductive outcomes of FET in patients with CE, especially those with RIF.
Similar content being viewed by others
Introduction
Chronic endometritis (CE) is characterized by persistent inflammation of the endometrium, presenting either asymptomatically or with subtle symptoms such as pelvic pain, abnormal uterine bleeding, dyspareunia, and leucorrhea [1, 2]. The histological diagnosis of CE relies on the presence of plasma cell infiltration in the endometrial stroma, with immunohistochemical (IHC) staining for syndecan-1 (CD138) being the primary diagnostic method [3]. CD138 is a transmembrane heparan sulfate proteoglycan expressed on plasma cell surface [4]. Hysteroscopy is a biopsy technique that aids in diagnosing intrauterine lesions, in which CE is suspected based on observations such as endometrial micro-polyps (typically 1–2 mm in diameter), focal or diffuse hyperemia, and stromal edema [5].
Prior studies have revealed associations between CE and adverse reproductive outcomes, including infertility, recurrent implantation failure (RIF), and recurrent pregnancy loss (RPL) [6,7,8,9]. Although CD138 staining has been widely used for CE diagnosis, understanding relevant clinical characteristics has gained importance for the diagnosis and treatment of CE. For instance, Takebayashi et al. demonstrated a higher CE diagnosis rate in women with endometriosis than in those without endometriosis (52.94% vs. 27.02%) [10]. Moreover, blockage of the fallopian tubes and hydrosalpinx have been linked to an increased incidence of CE [11, 12]. However, large-sample cohort studies comparing detailed medical histories of patients with and without CE are lacking.
Antibiotic treatment for CE has shown promise in eliminating plasma cells from the endometrial stroma, although most treatment regimens have been experimental, such as doxycycline and levofloxacin combined with metronidazole [13,14,15]. Cohort studies have indicated higher pregnancy and live birth rates in women cured of CE with antibiotic treatment than in those without CE or with persistent CE [8, 9, 16]. However, conflicting data exist, suggesting a potential increased risk of spontaneous abortion following antibiotics-cured CE among women after the initial embryo transfer (ET) [17]. Therefore, we focused on the clinical pregnancy status of frozen-thawed embryo transfer (FET) cycles within 1 year after antibiotic treatment to evaluate its impact on in-vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) reproductive outcomes. Besides, to the best of our knowledge, no studies have explored whether antibiotic treatment improves reproductive outcomes in women negative for CE on histology but positive for CE on hysteroscopy.
The retrospective study aimed to analyze the association between relevant clinical parameters and CE and investigate FET reproductive outcomes in both the CE and non-CE groups by conducting stratified analysis based on whether they were combined with RIF. Furthermore, we also aimed to determine whether antibiotic treatment is warranted for patients negative for CE at histology but positive for CE at hysteroscopy.
Materials and methods
Study design
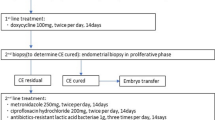
We conducted a retrospective cohort study at the Women’s Hospital of Nanjing Medical University from July 2020 to December 2021. Women underwent hysteroscopy and endometrial biopsy as routine procedures to histopathologically examine CD138 staining for diagnosing CE. Hysteroscopy was performed in cases of ET failure, recurrent spontaneous abortion, infertility, a history of tuberculosis infection, and abnormal uterine findings such as endometrial polyps, intrauterine adhesions (IUA), and congenital uterine malformations. This study was approved by the medical ethics committee of Nanjing Maternity and Child Health Care Hospital (NJFY-2021KY-110).
Study population
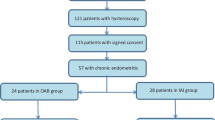
A total of 1352 women referred for hysteroscopy, endometrial biopsy, and CD138 IHC analysis for CE diagnosis were included. Exclusion criteria comprised those who had not undergone IVF-ET at our department, received antibiotic treatment within 3 months before hysteroscopy, and had incomplete medical history data. The analysis included 1088 women. Initially, all 1088 patients were part of the first analysis to identify the risk factors for CE.
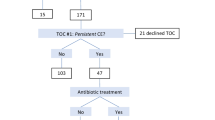
Subsequently, to assess the impact of CE on reproductive outcomes after antibiotic treatment, we included patients who underwent the FET cycle within 6 months after hysteroscopy. Exclusion criteria for this part were maternal age > 40 years, presence of other intrauterine lesions (e.g., IUA, submucosal myoma, and uterine malformation), chromosomal abnormalities in either spouse, and missing cycle data or follow-up. A total of 366 patients formed the second part to investigate pregnancy outcomes in infertile women with and without CE following FET cycles after hysteroscopy. Based on histological results, the patients were categorized into CE at histology (n = 112) and no CE at histology (n = 254). Patients with no CE at histology were further divided into CE at hysteroscopy (n = 133) and no CE at hysteroscopy (n = 121). Furthermore, those with no CE at histology but CE at hysteroscopy were divided into antibiotic treatment (n = 95) and control (n = 38) groups based on whether they received antibiotics. Finally, patients with CE at histology treated with antibiotics were classified as the CE group (n = 112), and those with no CE at histology not treated with antibiotics were classified as the non-CE group (n = 159). Subsequent FET reproductive outcomes were compared between the CE and non-CE groups, with further stratified analysis based on whether they were combined with RIF. The study flowchart is illustrated in Fig. 1.
Data collection
We searched the electronic medical database for data retrieval, including 1) maternal characteristics (maternal age, body mass index [BMI], gravidity, parity, main cause of infertility, duration of infertility, and maternal smoking history); 2) treatments and pregnancy outcomes of FET (number of ET procedures, good quality ET, blastocyst rate, insemination methods, stimulation protocol for FET, and endometrial thickness); 3) hysteroscopic, histological, and CD138 IHC examination results; and 4) maternal gynecologic and medical histories (dysmenorrhea, hydrosalpinx, endometriosis, polycystic ovarian syndrome, intrauterine device [IUD] use, pelvic inflammatory disease, endometrial polyps, IUA, tuberculosis, uterine cavity surgery, pelvic surgery, cervical surgery).
Sample collection
Hysteroscopy was performed using a 30° rigid hysteroscope (Shenyang Shenda Medical Equipment Co. Ltd.) with an outer diameter of 6.4 mm during the follicular phase (3–7 days after menstruation) of the menstrual cycle by two experienced specialists. Saline (0.9%) was used for expansion of the uterine cavity up to 100 mmHg pressure. Hysteroscopic features of CE, such as endometrial micropolyps, hyperemia, and edema, were observed, and other intrauterine manifestations (IUA, endometrial polyps, submucosal myoma, uterine malformations) were also noted. Immediately after hysteroscopy, endometrial biopsy specimens were obtained with a curette from the endometrial congestion. In patients with a normal uterine cavity, the biopsy sample was collected around the center of the anterior endometrium. The endometrial specimens were fixed in 4% paraformaldehyde and sent to the laboratory for histological and CD138 IHC analysis.
Diagnosis of CE
According to the Delphi poll reported previously [5], to diagnose CE with hysteroscopy, at least one of the following criteria need to be fulfilled: 1) strawberry aspect: large areas of hyperemia with white central points; 2) focal or diffuse endometrial hyperemia; 3) micro-polyps (< 1 mm in size with a distinct connective–vascular axis); 4) stromal edema (Figure S1). All endometrial biopsy specimens were analyzed by two experienced gynecologic pathologists at the Department of Pathology at Nanjing Maternity and Child Health Care Hospital. The plasma cell membrane showed strong positive CD138 staining, while the cytoplasm showed weak positive CD138 staining. The nuclei were rounded and localized to one side, and the thick chromatin was radially arranged along the nuclear membrane to form a wheel shape. After scanning the whole section at a lower magnification, the number of plasma cells was counted at 400 × magnification under the view with the greatest number of cells. According to published criteria [6, 18], the presence of one or more plasma cells in the endometrial stroma per 10 high-power fields (HPF) was considered the gold standard to diagnose CE by CD138 IHC staining.
Treatment and follow-up of CE
Women with confirmed CE by CD138 IHC staining were randomly administered oral doxycycline (100 mg twice per day for 14 days) or oral levofloxacin (500 mg twice per day for 14 days) combined with oral metronidazole (200 mg twice per day for 14 days). Whether women negative for CE on CD138 IHC staining but positive on hysteroscopy received antibiotic treatment was determined by the clinician on a case-by-case basis or based on the patient's preferences. Women negative for CE on both CD138 IHC staining and hysteroscopy did not receive antibiotic treatment.
Among the CE patients (n = 112) evaluated in this study for pregnancy outcomes, those with a plasma cell count below 10 per 10 HPFs (n = 104) did not require a repeat biopsy following antibiotic treatment. Conversely, patients with a plasma cell count exceeding 10 per 10 HPFs (n = 8) underwent a repeat biopsy to confirm the resolution of CE prior to embryo transfer. Notably, patients who persisted with CE despite repeat antibiotic therapy were excluded from embryo transfer and consequently from the analysis of pregnancy outcomes in this study.
FET protocol, ET, and luteal phase support (LPS)
The endometrial preparation protocol for FET, including the natural/stimulated cycle and the artificial cycle, was dependent on the characteristics and preferences of each woman. In the natural cycle, follicular size and endometrial thickness were monitored by transvaginal ultrasound from days 10 to 12 of the menstrual cycle. When the follicular diameter was ≥ 18 mm, progesterone concentration was ≤ 1.5 ng/mL, and endometrial thickness was ≥ 7 mm, an intramuscular injection of 10,000 IU human chorionic gonadotropin (hCG; Lizhu, China) was administered. Ovulation was detected after 24–48 h, and dydrogesterone (10 mg three times per day; Abbott Biologicals B.V., the Netherlands) was administrated orally from one day after ovulation to 14 days after FET. In the artificial cycle, the patients received 4 or 6 mg of oral estrogen per day (estradiol valerate [Progynova]; Bayer, France) on the second or third day of the menstrual cycle for 1 week. According to the endometrial thickness and the concentration of serum estradiol, the dose of estrogen was adjusted to 8–10 mg per day. In the downregulated artificial cycles, a gonadotropin-releasing hormone agonist (triptorelin acetate [Diphereline]; IPSEN, France) was administered to patients on days 2–4 of the menstrual cycle. Treatment with 4–6 mg of oral estrogen was initiated when the estradiol concentration was > 30 pg/ml, luteinizing hormone and follicle-stimulating hormone concentrations were < 5 IU/L, and endometrial thickness was < 5 mm. When the endometrial thickness reached 7 mm, serum estradiol concentration peaked at 200 pg/ml, and serum progesterone concentration was < 1.5 ng/ml, women were prescribed oral dydrogesterone (10 mg three times per day) combined with progesterone vaginal sustained-release gel (90 mg once per day) (Crinone; Merck Serono, UK). The vitrification process was performed for embryo freezing and resuscitation. Cleavage stage embryos and blastocysts were transferred on the fourth (P + 3) and sixth (P + 5) days after the day of progesterone exposure, respectively. Embryos were vitrified and warmed based on previously described protocols [19]. Cleavage stage embryos with at least six blastomeres, fragmentation of < 20%, and no obvious morphological abnormalities were defined as good quality embryos. Good quality blastocysts were scored as larger than 3BB (grades 3–6 AA/AB/BA/BB) according to the cavity expansion level, along with the inner cell mass and trophectoderm. One or two thawed embryos were transferred depending on the age, BMI, embryo quality, uterine condition (scarred uterine, uterine malformations, etc.), and personal will of each subject. Exogenous estrogen and progesterone administration lasted until 10 weeks of gestation if pregnancy was achieved.
Outcomes and definitions
The serum β-hCG test was performed 2 weeks after FET. LPS continued until 8–10 weeks of gestation when the patient had a positive pregnancy test. Clinical pregnancy was defined as presence of the intrauterine gestational sac with or without a fetal heartbeat, which was observed through transvaginal ultrasound after 6 weeks of gestation. The implantation rate was calculated as the number of gestational sacs divided by the number of embryos transferred. The early miscarriage was defined as pregnancy loss before 12 weeks of gestation. The late miscarriage was defined as pregnancy loss after 12 weeks but before 28 weeks of gestation. The RIF was defined as clinical pregnancy failure after four good quality embryos, with at least three fresh or frozen IVF cycles [20].
Statistical analysis
SPSS software, version 26.0, was used for statistical analyses. Continuous variables are presented as median with interquartile range (for non-normally distributed data), and categorical variables as a percentage (number/total number). The non-parametric Mann–Whitney U test was used to compare the mean age, BMI, gravidity, parity, number of abortions, number of uterine cavity surgeries, number of pelvic surgeries, duration of infertility, number of good quality embryos transferred, and endometrial thickness between the groups. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test, when necessary. A multivariate logistic regression analysis was performed to examine the independent effect of clinical characteristics on CE. Variables with significance in univariate analysis (P < 0.20) were entered into the multivariate logistic regression model, including history of parity ≥ 2, history of ectopic pregnancy, moderate-to-severe dysmenorrhea, hydrosalpinx, polycystic ovary syndrome (PCOS), endometrial polyps, number of uterine operations ≥ 2, and history of pelvic surgery. The crude odds ratio (OR) and adjusted OR (aOR) with 95% confidence intervals (CIs) were derived. Kaplan–Meier survival analysis was used to compare clinical pregnancy time between groups, with an observation time from hysteroscopy to the first clinical pregnancy outcome after hysteroscopy and a 1-year follow-up. A significance level of P < 0.05 was considered.
Results
Association between CE and clinical parameters
The association between clinical parameters in infertile women and the pathogenesis of CE was examined. Univariate analysis revealed that women with CE exhibited higher prevalence in parity ≥ 2 (4.1% vs. 1.9%, P = 0.029), history of ectopic pregnancy (12.9% vs. 8.5%, P = 0.021), moderate-to-severe dysmenorrhea (9.3% vs. 2.0%, P < 0.001), hydrosalpinx (10.2% vs. 5.9%, P = 0.009), endometrial polyps (42.7% vs. 36.7%, P = 0.049), number of uterine operations ≥ 2 (25.3% vs. 17.2%, P = 0.001), RIF (14.9% vs. 10.2%, P = 0.021) and No. of previous implantation failures (0.6 ± 1.0 vs. 0.5 ± 0.7, P < 0.001) than those without CE. Gravidity ≥ 2, history of smoking, endometriosis, adenomyosis, PCOS, history of intrauterine device use, pelvic inflammatory disease, IUA, and history of pelvic or cervical surgery showed no significant difference between women with and without CE (Table 1). After adjusting for significant variables from univariate analysis, the results confirmed that parity ≥ 2 (P = 0.022, aOR 2.466, 95% CI 1.140–5.334), history of ectopic pregnancy (P = 0.019, aOR 1.683, 95% CI 1.089–2.602), moderate-to-severe dysmenorrhea (P < 0.001, aOR 4.778, 95% CI 2.498–9.138), hydrosalpinx (P = 0.017, aOR 1.809, 95% CI 1.113–2.938), endometrial polyp (P = 0.012, aOR 1.394, 95% CI 1.076–1.805), number of uterine operations ≥ 2 (P = 0.011, aOR 1.495, 95% CI 1.096–2.041), and RIF (P = 0.004, aOR 1.723, 95% CI 1.184–2.507) were significantly associated with an increased risk of CE (Table 2).
Pregnancy outcomes in women with and without CE
A total of 366 out of 1088 women who underwent the first FET cycle within 6 months after hysteroscopy met the inclusion criteria. Among these, 112 women were positive for CE on histology, 133 were negative for CE on histology but positive on hysteroscopy, and 121 were negative for CE on both hysteroscopy and histology. Among those negative for CE on histology but positive on hysteroscopy, 95 received antibiotics, and the remaining 38 received no treatment.
Pregnancy outcomes of the first FET after hysteroscopy were analyzed in patients with CE after antibiotic treatment (CE group, n = 112) and non-CE patients without treatment (non-CE group, n = 159). Baseline characteristics were similar between the CE and non-CE groups (P > 0.05; Table 3). The CE group showed significantly lower miscarriage rates (8.7% vs. 20.2%, P = 0.045) and early miscarriage rates (2.9% vs. 16.8%, P = 0.005) than the non-CE group, with a significantly higher singleton live birth rate (45.5% vs. 32.7%, P = 0.032; Table 4). Additionally, the impact of antibiotic treatment on FET reproductive outcomes in patients negative for CE on histology but positive on hysteroscopy was analyzed. Among 133 women in this category, 95 received antibiotics (treatment group) and 37 did not (control group). Baseline characteristics were similar between the treatment and control groups (Table S1). Implantation rate (44.4% vs. 36.4%), clinical pregnancy rate (54.7% vs. 54.3%), miscarriage rate (11.5% vs. 15.8%), and live birth rate (48.4% vs. 42.1%) were comparable between these groups (P > 0.05; Table S2).
To assess the impact of CE after antibiotic treatment on clinical pregnancy outcomes, Kaplan–Meier curves were plotted from hysteroscopy to the first clinical pregnancy outcome, with a 1-year follow-up. The log-rank test indicated a statistically significant difference in the time from hysteroscopy to clinical pregnancy between the CE and non-CE groups [χ2 = 9.601, P = 0.0019, hazard ratio (HR) 1.257, 95% CI 1.058–1.495] (Fig. 2A). Further stratification into the RIF and non-RIF groups showed that RIF/CE patients after treatment had a significantly higher chance of achieving a clinical pregnancy than RIF/non-CE patients (χ2 = 9.471, P = 0.0021, HR 1.996, 95% CI 1.151–3.464; Fig. 2B). Chances for achieving a clinical pregnancy were similar between non-RIF/CE and non-RIF/non-CE women (χ2 = 2.792, P = 0.0947, HR 1.139, 95% CI 0.949–1.368; Fig. 2C).
Kaplan–Meier survival analysis of time to clinical pregnancy. The observation time was from hysteroscopy to the first clinical pregnancy outcome after hysteroscopy. A Kaplan–Meier survival analysis of clinical pregnancies in post-treatment CE and untreated non-CE patients; B Survival Analysis of Clinical Pregnancy between RIF/CE Patients After Treatment and Untreated RIF/Non-CE patients; and C Survival analysis of clinical pregnancy between non-RIF/CE patients and untreated non-RIF/non-CE patients after treatment
Discussion
This retrospective cohort study reported significant associations between specific clinical factors and the occurrence of CE. Notably, a history of parity ≥ 2, hydrosalpinx, history of ectopic pregnancy, moderate-to-severe dysmenorrhea, endometrial polyps, a history of uterine operations ≥ 2, and RIF were identified as relevant risk factors for CE. Furthermore, antibiotic treatment for CE was found to correlate with improved reproductive outcomes, including a reduced miscarriage rate and an increased singleton live birth rate in the first FET cycle. The benefits extended to a higher chance of clinical pregnancy within 1 year after hysteroscopy, particularly among patients with a history of RIF.
To more comprehensively assess the risk factors for CE, we collected detailed medical history of the patients, including gynecologic and obstetric history. The analysis revealed that hydrosalpinx significantly increased the likelihood of CE. Hydrosalpinx-induced toxic fluid backflow into the uterine cavity may compromise embryo viability and diminish endometrial receptivity [21, 22]. Elevated expression of endometrial interleukin (IL)-2 and tumor necrosis factor (TNF)-α, indicative of an inflammatory response, may contribute to this phenomenon [19]. Chronic inflammation, with its potential to disseminate through blood and lymphatic circulation, provides a plausible explanation for the elevated risk of CE and adverse pregnancy outcomes in women with hydrosalpinx. Notably, our study revealed an association between CE and a history of ectopic pregnancy. Ectopic pregnancies often result in fallopian tube damage, primarily due to infection, leading to a six-fold increase in inflammation compared with that in healthy fallopian tubes [23]. Our findings align with existing research linking CE to tubal pathologies [11, 12]. The female reproductive tract forms a continuum of microbiotas that change from the vagina to the ovaries [24], leading to an interplay between fallopian tube inflammation and CE pathogen infection; this interaction impedes ET from the fallopian tube to the endometrium, thereby contributing to an elevated risk of ectopic pregnancy.
Moreover, our results suggest an association between CE and moderate-to-severe dysmenorrhea (aOR 4.588, P < 0.001). Previous studies have elucidated dysmenorrhea as a complex process influenced by factors such as inflammatory cytokine concentration and uterine contractile activity [25,26,27]. In a case–control study by Ma et al., genes encoding pro-inflammatory cytokines—TNF-α, IL-1β, IL-6, and IL-8—were upregulated in primary dysmenorrhea [28]. These cytokines facilitate the synthesis and release of prostaglandin F2α and oxytocin, which result in increased uterine contraction, reduced endometrial blood flow, and menstrual pain [29, 30]. The observed elevation in pro-inflammatory cytokine levels in CE may contribute to increased levels of PGE2α and oxytocin, providing a plausible explanation for the higher incidence of dysmenorrhea in women with CE.
A retrospective study identified multiparity as an independent risk factor for CE [16], and our investigation aligns with these findings, revealing that parity ≥ 2 (aOR 2.338, P = 0.030) and number of uterine operations ≥ 2 (aOR 1.524, P = 0.008) significantly elevate the risk of CE. The delivery process and uterine cavity surgery create connections between the uterine cavity and the external environment, allowing pathogenic bacteria to ascend through the reproductive tract into the uterine cavity and trigger CE. Contributing factors may include compromised patient immunity, surgical instrument contamination, and infection during surgery.
Our study indicated that patients with CE who underwent antibiotic treatment experienced significantly lower miscarriage rates (8.7% vs. 20.2%) and early miscarriage rates (2.9% vs. 16.8%) than untreated non-CE patients. Conversely, the singleton live birth rate was notably higher in the treated CE group (45.5% vs. 32.7%), aligning with findings from Kitaya et al. and Vitagliano et al. [15, 16]. However, antibiotic treatment for patients with RIF and its impact on subsequent IVF cycles remains of interest to clinicians due to conflicting reported data. Some studies have reported significantly higher clinical pregnancy and live birth rates after antibiotic treatment for CE patients with RIF [9, 16], whereas others reported similar outcomes between CE and non-CE patients with RIF [31, 32]. The previous analysis of CE related risk factors showed that RIF and the No. of previous implantation failure are related to the occurrence of CE. Besides, our Kaplan–Meier survival analysis, stratified by RIF, revealed that treated RIF/CE patients were more likely to achieve clinical pregnancy within 1 year after hysteroscopy than untreated non-CE patients. Conversely, patients without RIF exhibited similar chances for clinical pregnancy regardless of antibiotic treatment. This is probably because patients with RIF have a higher prevalence of CE, and antibiotic treatment may be particularly beneficial for women with CE and RIF, emphasizing its efficacy in improving pregnancy outcomes, especially for infertile women with RIF.
To the best of our knowledge, no studies have examined the effectiveness of antibiotic treatment in women positive for CE on hysteroscopy but negative on histology, making this the first such report. Considering the fertility desire of women undergoing IVF-ET, whether antibiotic treatment is needed in women who are negative for CE on CD138 IHC staining but positive for CE on hysteroscopy merits attention. In this study, our findings indicate no significant differences in pregnancy outcomes between the treatment and control groups. This implies that antibiotic treatment may be unnecessary for women negative for CE on CD138 IHC staining but positive on hysteroscopy. Moreover, there lacks a guideline for antibiotic administration in cases with positive for CE on hysteroscopy but negative on histology. Given the constraints of our limited sample size and subjective approach to medication usage driven by physician and patient discretion, we are unable to offer compelling evidence. Future endeavors will likely require prospective studies with larger sample sizes to furnish more robust evidence.
One particular strength of our study is that it is the first clinical study to focus on differences in the time to clinical pregnancy in subsequent FET cycles between CE patients treated with antibiotics and non-CE patients. Despite our efforts, the present study is not without limitations. First, this was a single-center retrospective study with a small sample size, which restricts the generalizability of our findings. Second, patients were not screened for autoimmune diseases, because these diseases may influence the pregnancy outcomes. Finally, molecular microbial analysis was not employed, which may be a more accurate method and may replace histology as the gold standard in CE diagnosis in the future. Future prospective randomized controlled studies with larger sample sizes are warranted to validate and refine the findings of this retrospective study.
Conclusion
In conclusion, CE, characterized by plasma cell infiltration, is associated with factors such as a history of parity ≥ 2, hydrosalpinx, history of ectopic pregnancy, moderate-to-severe dysmenorrhea, endometrial polyps, a history of uterine operations ≥ 2, and RIF. Encouragingly, infertile women with CE, especially those with RIF, demonstrated positive reproductive outcomes in subsequent FET cycles following oral antibiotic treatment. To validate our findings, further randomized controlled studies are imperative.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zargar M, Ghafourian M, Nikbakht R, et al. Evaluating Chronic Endometritis in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss by Hysteroscopy and Immunohistochemistry. J Minim Invasive Gynecol. 2020;27(1):116–21.
Cicinelli E, Trojano G, Mastromauro M, et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil Steril. 2017;108(2):289–95.e1.
Kitaya K, Matsubayashi H, Yamaguchi K, et al. Chronic Endometritis: Potential Cause of Infertility and Obstetric and Neonatal Complications. Am J Reprod Immunol. 2016;75(1):13–22.
Bayer-Garner IB, Nickell JA, Korourian S. Routine syndecan-1 immunohistochemistry aids in the diagnosis of chronic endometritis. Arch Pathol Lab Med. 2004;128(9):1000–3.
Cicinelli E, Vitagliano A, Kumar A, et al. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: proposal and reliability evaluation through an international randomized-controlled observer study. Fertil Steril. 2019;112(1):162–73.e2.
Song D, Feng X, Zhang Q, et al. Prevalence and confounders of chronic endometritis in premenopausal women with abnormal bleeding or reproductive failure. Reprod Biomed Online. 2018;36(1):78–83.
Kitaya K. Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011;95(3):1156–8.
McQueen DB, Bernardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil Steril. 2014;101(4):1026–30.
Cicinelli E, Matteo M, Tinelli R, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323–30.
Takebayashi A, Kimura F, Kishi Y, et al. The association between endometriosis and chronic endometritis. PLoS ONE. 2014;9(2):e88354.
Holzer I, Ott J, Kurz C, et al. Is Chronic Endometritis Associated with Tubal Infertility? A Prospective Cohort Study. J Minim Invasive Gynecol. 2021;28(11):1876–81.
Peng J, Guo F, Liu H, et al. Correlation between hysteroscopy findings in patients with hydrosalpinx and chronic endometritis. Int J Gynaecol Obstet. 2022;157(2):471–5.
Cicinelli E, Matteo M, Trojano G, et al. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. 2018;79(1):e12782.
Haggerty CL, Ness RB, Amortegui A, et al. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol. 2003;188(1):141–8.
Vitagliano A, Saccardi C, Noventa M, et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil Steril. 2018;110(1):103–12.e1.
Kitaya K, Matsubayashi H, Takaya Y, et al. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol. 2017;78(5):e12719.
Duan H, Li X, Hao Y, et al. Risk of spontaneous abortion after antibiotic therapy for chronic endometritis before in vitro fertilization and intracytoplasmic sperm injection stimulation. Fertil Steril. 2022;118(2):337–46.
Song D, Li TC, Zhang Y, et al. Correlation between hysteroscopy findings and chronic endometritis. Fertil Steril. 2019;111(4):772–9.
Ji H, Zhou Y, Cao S, et al. Effect of embryo developmental stage, morphological grading, and ploidy status on live birth rate in frozen cycles of single blastocyst transfer. Reprod Sci. 2021;28(4):1079–91.
Cimadomo D, Craciunas L, Vermeulen N, et al. Definition, diagnostic and therapeutic options in recurrent implantation failure: an international survey of clinicians and embryologists. Hum Reprod. 2021;36(2):305–17.
Mukherjee T, Copperman AB, McCaffrey C, et al. Hydrosalpinx fluid has embryotoxic effects on murine embryogenesis: a case for prophylactic salpingectomy. Fertil Steril. 1996;66(5):851–3.
Roberts JE, Clarke HJ, Tulandi T, et al. Effects of hydrosalpingeal fluid on murine embryo development and implantation. J Assist Reprod Genet. 1999;16(8):421–4.
Marion LL, Meeks GR. Ectopic pregnancy: History, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012;55(2):376–86.
Chen C, Song X, Wei W, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8(1):875.
Hailemeskel S, Demissie A, Assefa N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in Ethiopia. Int J Womens Health. 2016;8:489–96.
Bulletti C, de Ziegler D, Polli V, et al. Uterine contractility during the menstrual cycle. Hum Reprod. 2000;15(Suppl 1):81–9.
Halbert DR, Demers LM, Darnell Jones DE. Dysmenorrhea and prostaglandins. Obstet Gynecol Surv. 1976;31(1):77–81.
Ma H, Hong M, Duan J, et al. Altered cytokine gene expression in peripheral blood monocytes across the menstrual cycle in primary dysmenorrhea: a case-control study. PLoS ONE. 2013;8(2):e55200.
Skarzynski DJ, Miyamoto Y, Okuda K. Production of prostaglandin f(2alpha) by cultured bovine endometrial cells in response to tumor necrosis factor alpha: cell type specificity and intracellular mechanisms. Biol Reprod. 2000;62(5):1116–20.
Huang JC, Liu DY, Yadollahi S, et al. Interleukin-1 beta induces cyclooxygenase-2 gene expression in cultured endometrial stromal cells. J Clin Endocrinol Metab. 1998;83(2):538–41.
Johnston-MacAnanny EB, Hartnett J, Engmann LL, et al. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437–41.
Demirdag E, Guler I, Cevher Akdulum MF, et al. Subsequent IVF outcomes following antibiotic therapy for chronic endometritis in patients with recurrent implantation failure. J Obstet Gynaecol Res. 2021;47(12):4350–6.
Acknowledgements
We want to express our thanks to all patients and their partners, nurses, doctors, and other medical staff in the Reproductive Center and Obstetrics and Gynecology of Women’s Hospital of Nanjing Medical University for agreeing to participate in this study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by National Natural Science Foundation of China (grant nos. 81971386, 81871210 and 81701507).
Author information
Authors and Affiliations
Contributions
Study design was done by Ling XF, Shen R and Hua XD; data collection by Ni DY and Chen YT; statistical analysis by Xie QJ, Jiang W, and Li X; manuscript drafting by Xie QJ, and Zhao C; and research supervision by Li XL and Ling XF. All authors contributed to manuscript revision and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study adhered to Ethical Review Methods for Biomedical Research Involving Humans, the Declaration of Helsinki and the International Ethical Guidelines for Biomedical Research Involving Humans. This study retrospectively analyzed patient data anonymously, which eliminated the need for informed patient consent, and was approved by the medical ethics committee of Nanjing Maternity and Child Health Care Hospital (NJFY-2021KY-110).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, Q., Zhao, C., Jiang, W. et al. Antibiotics improve reproductive outcomes after frozen-thaw embryo transfer for chronic endometritis treatment, especially in those with repeated implantation failure. BMC Women's Health 24, 430 (2024). https://doi.org/10.1186/s12905-024-03274-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-03274-x