Abstract
Purpose
This review aims to sum up current knowledge on the sensitivity and specificity of ultrasound features suggestive of acute pelvic inflammatory disease (PID).
Methods
A PubMed database search was undertaken, using the MeSH terms “(pelvic inflammatory disease or salpingitis or adnexitis) and ultrasonography”. We included original articles evaluating the performance of vaginal ultrasound in detecting acute PID.
Results
Seven articles were selected, including between 18 and 77 patients each. The golden standard used was laparoscopy/endometrial biopsy in six studies and mostly clinical evaluation in one. “Thick tubal walls” proved to be a specific and sensitive ultrasound sign of acute PID, provided that the walls of the tubes can be evaluated, i.e., when fluid is present in the tubal lumen (100 % sensitivity). The cogwheel sign is also a specific sign of PID (95–99 % specificity), but it seems to be less sensitive (0–86 % sensitivity). Bilateral adnexal masses appearing either as small solid masses or as cystic masses with thick walls and possibly manifesting the cogwheel sign also seems to be a reasonably reliable sign (82 % sensitivity, 83 %specificity). Doppler results overlap too much between women with and without acute PID for them to be useful in the diagnosis of acute PID, even though acutely inflamed tubes are richly vascularized at color Doppler.
Conclusions
Even though the results of our review suggest that transvaginal ultrasound has limited ability to diagnose acute PID, it is likely to be helpful when managing women with symptoms of acute PID, because in some cases the typical ultrasound signs of acute PID can be detected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the progress in the last decade in medicine, acute pelvic inflammatory disease (PID) is still a challenge regarding diagnosis. In many hospitals diagnostic laparoscopy, the golden standard for the diagnosis of acute PID proposed by Jacobson and Westrom [1], is not used routinely because of the high costs and resources involved. Moreover, subtle inflammation of the tubes might not be detectable at laparoscopy [2]. Consequently, a diagnosis of acute PID is often based on clinical findings, which are unspecific and may lead to unnecessary use of antibiotics. This is because current guidelines for diagnosing and treating acute PID suggest a low threshold for initiating antibiotic treatment [3].
Ultrasound is a well-established tool for diagnosing pelvic pathology, and the sonographic appearance of diseased tubes has been described by several authors [4, 5]. This paper aims to sum up current knowledge on the sensitivity and specificity of transvaginal ultrasound features suggestive of acute PID.
Methods
This paper was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy
A PubMed database search was undertaken using the following combination of MeSH terms “(pelvic inflammatory disease or salpingitis or adnexitis) and ultrasonography”. Initially no time limit was set. The results contained articles from 1970 until March 2013. Using the database filters, only publications with an abstract in English and involving humans were included, and review articles were excluded. The titles of all remaining articles were reviewed and if relevant, the abstract was analyzed. If the abstract appeared to fulfill our inclusion criteria (see below), the full text article was read. Reference lists of original articles and some review articles were also examined for relevant publications.
Inclusion criteria
-
Full text in English.
-
Original article (i.e., case reports, letter to the editor, etc., were excluded).
-
Sensitivity and specificity of specified ultrasound features of acute PID involving the fallopian tubes (acute salpingitis, pyosalpinx, tubo-ovarian abscess) reported, or enough data presented for the authors of this review to calculate sensitivity and specificity themselves.
-
All patients examined with transvaginal ultrasound.
-
Inclusion of patients with a clinical suspicion of acute PID not related to a recent pregnancy or surgical procedure, minimal criteria of acute PID being acute pelvic pain of less than a month’s duration and at least one of the following: abdominal tenderness, palpable adnexal mass, pathological discharge, fever or laboratory findings indicating an acute inflammatory process.
-
Negative pregnancy test.
-
Inclusion of a control group (patients with a final diagnosis other than acute PID).
-
Initially, we had the intention to include only studies where a diagnosis of acute PID was confirmed by laparoscopy or laparotomy (gold standard), but because there were very few studies fulfilling these criteria, we accepted also studies using endometrial biopsy or a clinical diagnosis of acute PID as gold standard.
Data extraction
For each clinical study, the following data were extracted: study design, setting, number of participants, description of population characteristics, golden standard, ultrasound markers for salpingitis and their sensitivity and specificity with regard to acute tubal inflammatory pathology.
Results
The selection of the seven articles included in our review is outlined in Fig. 1. The selected articles describe prospective observational or case–control studies including between 18 and 77 patients examined with transvaginal ultrasound by sonographers or gynecologists. The characteristics of the studies are described in Table 1. The golden standard used for the diagnosis of acute PID was laparoscopy or endometrial biopsy in six of the seven studies. In one study, the diagnosis was based mostly on clinical evaluation with only 17 % of the patients undergoing surgical exploration [4].
Flow diagram illustrating our literature search and selection of original articles for inclusion in our review 1 PubMed database was searched using the combination of MeSH Terms “(pelvic inflammatory disease or salpingitis or adnexitis) and ultrasonography”, 2 reference lists of original articles and of some review articles, 3 for example, type of ultrasound unknown, or ultrasound features not specified
The data regarding gray-scale ultrasound features of acute tubal inflammation are presented in Table 2 and those regarding Doppler velocimetry in Table 3. All seven articles were used for evaluation of gray-scale ultrasound markers of salpingitis, two [5, 6] of them could be used for evaluation of Doppler findings.
Gray-scale ultrasound features reported to be associated with acute tubal inflammatory disease
Thick tubal walls
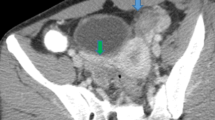
Inflammation causes swelling of the tubal mucosa, and if fluid representing exudate or pus (pyosalpinx) is present in the tubal lumen, the tubal wall thickness can be measured. Five studies [4–8] evaluated the diagnostic performance of thickened tubal wall as a sign of acutely inflamed tubes (Table 2). In all five studies, tubal wall thickness was a specific sign of acutely inflamed tubes (specificity 90–100 %). However, the definition of thickened tubal wall differs between studies: Timor-Tritsch et al. [4] and Molander et al. [6] used a cut-off of 5 mm to indicate thickened wall, while Patten et al. [7], Cacciatore et al. [8] and Romosan et al. [5] used the subjective evaluation of the ultrasound examiner. The sensitivity of thickened tubal walls varied between 29 and 100 %. Molander et al. [6] could not measure the tubal wall thickness in six of six patients with mild acute salpingitis (the tubes appearing as solid masses at ultrasound), but found thick walls in all patients with pyosalpinx or tubo-ovarian abscess. Romosan et al. [5] reported similar results: in all patients with a unilocular (four tubes), multilocular cystic (three tubes) or multilocular solid (three tubes) adnexal mass, confirmed at laparoscopy to correspond to an acutely inflamed tube, the walls of the mass were thick according to subjective evaluation by the ultrasound examiner; while in patients with solid adnexal masses (18 tubes) representing acute salpingitis confirmed at laparoscopy, the thickness of the tubal walls could not be evaluated. Using only those tubes that were judged to be fluid filled at ultrasound examination to calculate sensitivity, the sensitivity of thick tubal walls was 100 % in all four studies where there was information on whether the tubes were fluid filled or not, i.e., 14/14 [4], 10/10 [5], 14/14 [6] and 11/11 [8]. The ultrasound feature “thick tubal wall” is shown in Fig. 2.
Ultrasound images illustrating thick walls of fluid-filled acutely inflamed tubes. In both (a) and (b) the diagnosis is pyosalpinx. Note the thick incomplete septa present in both (a) and (b). In Fig. 4 showing an ultrasound image of hydrosalpinx, i.e., chronic tubal disease, the incomplete septum and the walls of the fluid-filled tube are thin
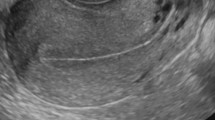
Cogwheel sign
The cogwheel sign was first described by Timor-Tritsch et al. [4]. It is defined as a cogwheel-shaped structure with sonolucent or echogenic cyst contents visible on a cross-section of a tube with swollen walls and swollen mucosal folds [4] (Fig. 3). Timor-Tritsch et al. found the cogwheel sign to be a both sensitive and specific sign of acutely inflamed tubes (Table 2). However, it was present in only 55 % of the patients with acutely inflamed tubes in the study of Molander et al. [6] (or in 79 % of those patients with acute PID where there was fluid in the tubal lumen at ultrasound) and was found in none of the 17 patients with acutely inflamed tubes in the study of Romosan et al. [5] (Table 2). The latter two studies used laparoscopy as golden standard; while in the study by Timor-Tritsch et al., the diagnosis of PID was based on clinical evaluation in most cases. The specificity of the cogwheel sign was high in all three studies reporting on its diagnostic performance [4–6].
Fluid in the pouch of Douglas
Free fluid in the pelvis, representing inflammatory exudate or pus, is easy to see and measure with vaginal ultrasound. Its reported sensitivity with regard to acute PID varies between 37 and 82 % and its specificity between 43 and 90 % (Table 2). The results in Table 2 show that it is not a reliable sign of acute PID.
Polycystic-like ovaries
The idea of using polycystic ovaries as a marker of acutely inflamed adnexa comes from the assumption that inflammation of the ovaries increases their volume by producing inflammatory exudate and edema resulting in increased volume of the ovarian stroma. Cacciatore et al. [8] suggested that “a thickened ovarian capsule might prevent normal follicular growth, thus causing multifollicular degeneration”. However, different studies use different definitions of “polycystic-like ovaries” as a sign of acute inflammation in the adnexa. Some [8, 9] used the criteria of Adams et al. [10], i.e., ten or more cysts 2–10 mm in diameter in each ovary. Boardman et al. [11] used the term “multicystic ovaries” and defined it as six or more cysts <10 mm in each ovary. Even though Cacciatore et al. [8] found polycystic ovaries in all patients with acute PID, polycystic or multicystic ovaries do not seem to be a reliable sign of acute tubal inflammation, see Table 2. Tukeva et al. [9] found polycystic-like ovaries at magnetic resonance imaging in 19 % (4/21) of women with early salpingitis, pyosalpinx or tubo-ovarian abscess, but they did not search for polycystic-like ovaries at ultrasound examination [9].
Adnexal masses
Romosan et al. found the best ultrasound marker for diagnosing acute salpingitis in women with clinical signs of acute PID to be the presence of bilateral adnexal masses or bilateral masses lying adjacent to the ovary. The ultrasound appearance of the 28 acutely inflamed tubes in that study was as follows: using the terminology of the International Ovarian Tumor Analysis (IOTA) group [12], 18 (64 %) masses were solid, 7 (25 %) were multilocular cystic, and 3 (11 %) were multilocular solid, and all cystic masses had thick walls [5]. They described the masses lying adjacent to the ovary as being most often solid, 2–3 cm in diameter and well vascularized at color Doppler. No other study reported the sensitivity and specificity of bilateral adnexal masses. In the study by Molander et al. [6], all six patients with mild salpingitis according to laparoscopy had ultrasound findings of “an echogenic and rather homogenous mass with indistinct margins close to the ovary” (Table 2).
Incomplete septa
Timor-Tritsch et al. [4] defined incomplete septa as “hyperechoic septa that originate as a triangular protrusion from one of the walls of a cystic lesion but not reaching the opposite wall” (Fig. 4). In the work of Timor-Tritsch et al. [4] this ultrasound feature was found in 86 % (12/14) of patients with acute symptoms of PID and also in 93 % (56/60) of the patients with a history of chronic tubal pathology. Molander et al. [6] reported similar results, incomplete septa being present at ultrasound examination in 60 % (12/20) of patients with acute PID (or in 86 % of those patients with acute PID where there was fluid in the tubal lumen at ultrasound) and in 85 % (17/20) of the control group with hydrosalpinx. Thus, the presence of incomplete septa is neither a sensitive nor specific ultrasound sign of acute salpingitis (Table 2).
Ultrasound image illustrating the ultrasound feature incomplete septum, i.e., a thin strand of tissue running across a cystic cavity from one internal surface to the contralateral side, but which is not complete in some scanning planes [12]. The diagnosis here is hydrosalpinx. Please note that both the walls and the incomplete septum are thin. This is in contrast to the ultrasound findings illustrated in Fig. 2 showing an acutely inflamed tube with thick walls and thick incomplete septae
Tubo-ovarian complex
The ultrasound term tubo-ovarian complex refers to an ultrasound image of agglutinated ovaries and tubes in a patient with clinical signs of acute PID where the ovaries and tubes can still be identified at ultrasound, but where the ovary cannot be separated from the tube by pushing on the lesion with the vaginal probe [4]. This condition may be regarded as a predecessor of tubo-ovarian abscess. Timor-Tritsch et al. [4] found this ultrasound feature to have a sensitivity with regard to acute PID of 36 % (5/14) and a specificity of 98 % (59/60), but the diagnosis of PID was based on clinical features in 64 of 74 patients (86 %), only 10 of the 74 patients with clinical signs of acute PID undergoing surgical exploration. Cacciatore et al. [8] found this sign in only 2 of 13 (15 %) patients with acute PID defined as plasma cell endometritis in endometrial biopsy, and in none of 38 patients without plasma cell endometritis (Table 2).
Total breakdown of the normal adnexal architecture with formation of a conglomerate where neither the ovary nor the tubes can be recognized as such: tubo-ovarian abscess
Total breakdown of the normal adnexal architecture with formation of a conglomerate where neither the ovary nor the tubes can be separately recognized as such indicates the presence of a tubo-ovarian abscess. The results of this marker with regard to acute PID in general are presented in Table 2. The sensitivity and specificity of this ultrasound finding with regard to tubo-ovarian abscess was 100 % (5/5) and 100 % (35/35) in the study of Molander et al. [6] and 50 % (4/8) and 86 % (18/22) in the study of Tukeva et al. [9] with four false-positive ultrasound results; i.e., at laparoscopy the true diagnoses were salpingitis in two cases, and endometrioma and tubal torsion in one case each. Patten et al. [7], presenting results per adnexum (two patients previously had unilateral salpingectomy) reported a sensitivity of 89 % (8/9) and a specificity of 96 % (24/25) with one false-positive ultrasound result where at laparoscopy a tube was convoluted and dilated, but not involved in an abscess. In all these three studies, reporting on the ultrasound feature “total breakdown of the normal adnexal architecture” the golden standard was laparoscopy sometimes followed by laparotomy.
Doppler ultrasound features reported to be associated with acute tubal inflammatory disease
Doppler velocimetry
Tissue inflammation is accompanied by increased blood flow, vasodilatation and angiogenesis [13]. In women with a clinical diagnosis of acute PID, changes in blood flow velocities in the pelvic vessels before, during and after antibiotic treatment have been observed by performing serial spectral Doppler ultrasound examinations. In the acute phase of the infection, the vascular resistance (pulsatility index, resistance index) in the uterine arteries [14, 15], tubo-uterine and ovarian arteries [15] and arteries of pelvic masses [16] were low, but returned to normal when the infection subsided [14–16].
Two studies included in our review reported results of Doppler velocimetry in patients with acute PID and in a control group [5, 6] (Table 3). Molander et al. [6] found significantly lower vascular resistance in blood vessels in the tubal walls and adnexal masses of women with acute PID than in a control group of women with hydrosalpinx, but they concluded that the results overlapped too much for them to be clinically useful in isolation (Table 3). Romosan et al. [5] found lower vascular resistance and higher blood flow velocities in the uterine and tubal arteries of patients with salpingitis than in patients with other diagnoses, but their results, too, overlapped too much between patients with different diagnoses for them to be clinically useful (Table 3).
Color content of adnexal mass
Two of the studies included in this review evaluated the color content of adnexal masses using subjective interpretation of the color Doppler image by the ultrasound examiner. Molander et al. [6] defined hyperemia as a large number of vessels (arteries and veins) at power Doppler examination with low impedance arterial blood flow. They found hyperemia so defined in all patients with acute salpingitis (20/20), but only in 2 of 20 (10 %) control patients with hydrosalpinx. Romosan et al. used a visual analog scale (VAS) graded from 0 to 100 to estimate the color content of any adnexal mass found. They reported that inflamed tubes were very richly vascularized at color Doppler examination, but in their study where all patients had clinical signs of acute PID, the results overlapped substantially between patients with and without acutely inflamed tubes [5].
Discussion/summary of evidence
We have reviewed and summarized the extremely scarce scientific evidence on the sensitivity and specificity of specific ultrasound features with regard to discriminating between acute PID and other conditions in the pelvis. It seems that “thick tubal walls” is a specific and reasonably sensitive ultrasound sign of acute PID with tubal involvement provided that the walls of the tubes can be evaluated, i.e., when fluid is present in the tubal lumen. The cogwheel sign is also a specific sign of acute PID, but it seems to be less sensitive than “thick tubal walls”. Fluid in the pouch of Douglas, which is a physiologic phenomenon in asymptomatic women [17] and polycystic-like ovaries are not helpful for discriminating between acute PID and other conditions, and incomplete septa, which are typical of diseased tubes in general [4, 6] do not help discriminating between acute and chronic tubal pathology because they are present in both conditions [4, 6]. The presence of bilateral adnexal masses or bilateral masses adjacent to the ovary at transvaginal ultrasound seems to be a reasonably reliable sign of acute salpingitis, the adnexal masses appearing either as small solid masses or as cystic masses with thick walls and possibly manifesting the cogwheel sign. Total breakdown of the normal adnexal architecture with formation of a mass is a sensitive and specific marker for diagnosing tubo-ovarian abscesses, but not for diagnosing PID in general.
Even though acutely inflamed tubes are richly vascularized at color Doppler [5, 6, 14–16], our review shows that spectral Doppler results overlap too much between women with and without acute PID for them to be useful in isolation in the diagnosis of acute PID [5, 6]. Color/power Doppler findings on the other hand might be useful for discriminating between acute and chronic PID, acutely inflamed tubes being more richly vascularized at power Doppler than chronic hydrosalpinges [6]. However, in women presenting with clinical signs of acute PID, color Doppler findings seem to overlap too much between women with acute PID and other pathological conditions in the pelvis for them to be clinically useful [5].
We deliberately did not include chronic tubal disease in our review (even though in two of the studies in our review, chronic tubal pathology was used as control [4, 6]). This is because we wanted to get a view of how helpful ultrasound can be for confirming or refuting a clinical suspicion of acute PID, because this is a clinical problem. The typical ultrasound features of chronic tubal disease have been nicely described by Timor-Tritsch et al. [4] and include “pear-shaped, ovoid, or retort-shaped fluid-filled structure”, “thin walls”, “incomplete septa” and the highly specific ultrasound feature “beads on a string” (Fig. 5).
To the best of our knowledge, our review is the first to sum up the scientific evidence concerning the sensitivity and specificity of specific ultrasound features with regard to acute PID. Unfortunately the evidence is very scarce. In all likelihood, this is explained by the difficulty of carrying out studies aiming at estimating the sensitivity and specificity of ultrasound with regard to acute PID. First, there is no obvious gold standard, but we believe that the best gold standard is diagnostic laparoscopy. Second, a clinically relevant study should be carried out exclusively among women with clinical signs of acute PID. Only three of the studies in our review had a study design where all women included had clinical signs of PID and where all women underwent diagnostic laparoscopy [5, 7, 9]. Ideally, however, the diagnosis of salpingitis should be confirmed both with cultures from the tube and with biopsy of the fimbriae of the tube [2], but this was not done in any of the studies in our review. Third, the sensitivity and specificity of specific ultrasound features with regard to acute PID is highly dependent on the severity of the disease in the population studied.
As many as 30–40 % of patients presenting with symptoms and clinical signs of acute PID have a true diagnosis completely unrelated to genital infection, e.g., appendicitis, endometriosis, adnexal torsion, or ovarian cysts [1, 5, 6, 9, 18]. Even though the results of our review suggest that transvaginal ultrasound has limited ability to diagnose acute PID, it is likely to be helpful when managing women with symptoms and clinical signs of acute PID, because in some cases the typical ultrasound signs of acute PID may be detected.
References
Jacobson L, Westrom L (1969) Objectivized diagnosis of acute pelvic inflammatory disease. Diagnostic and prognostic value of routine laparoscopy. Am J Obstet Gynecol 105(7):1088–1098. doi:0002-9378(69)90132-X
Sellors J, Mahony J, Goldsmith C, Rath D, Mander R, Hunter B, Taylor C, Groves D, Richardson H, Chernesky M (1991) The accuracy of clinical findings and laparoscopy in pelvic inflammatory disease. Am J Obstet Gynecol 164(1 Pt 1):113–120
Centers for Disease Control and Prevention (2010) Sexually transmitted diseases treatment guidelines. MMWR 59(RR-12):63–67
Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS (1998) Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol 12(1):56–66. doi:10.1046/j.1469-0705.1998.12010056.x
Romosan G, Bjartling C, Skoog L, Valentin L (2013) Ultrasound for diagnosing acute salpingitis: a prospective observational diagnostic study. Hum Reprod 28(6):1569–1579. doi:10.1093/humrep/det065
Molander P, Sjoberg J, Paavonen J, Cacciatore B (2001) Transvaginal power Doppler findings in laparoscopically proven acute pelvic inflammatory disease. Ultrasound Obstet Gynecol 17(3):233–238. doi:10.1046/j.1469-0705.2001.00353.x
Patten RM, Vincent LM, Wolner-Hanssen P, Thorpe E Jr (1990) Pelvic inflammatory disease. Endovaginal sonography with laparoscopic correlation. J Ultrasound Med 9(12):681–689
Cacciatore B, Leminen A, Ingman-Friberg S, Ylostalo P, Paavonen J (1992) Transvaginal sonographic findings in ambulatory patients with suspected pelvic inflammatory disease. Obstet Gynecol 80(6):912–916
Tukeva TA, Aronen HJ, Karjalainen PT, Molander P, Paavonen T, Paavonen J (1999) MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology 210(1):209–216
Adams J, Polson DW, Franks S (1986) Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 293(6543):355–359
Boardman LA, Peipert JF, Brody JM, Cooper AS, Sung J (1997) Endovaginal sonography for the diagnosis of upper genital tract infection. Obstet Gynecol 90(1):54–57. doi:10.1016/S0029-7844(97)00241-X
Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I (2000) Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol 16(5):500–505. doi:10.1046/j.1469-0705.2000.00287
Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R (2011) Circulating fibrocytes––biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol 291:1–19. doi:10.1016/B978-0-12-386035-4.00001-X
Ozbay K, Deveci S (2011) Relationships between transvaginal colour Doppler findings, infectious parameters and visual analogue scale scores in patients with mild acute pelvic inflammatory disease. Eur J Obstet Gynecol Reprod Biol 156(1):105–108. doi:10.1016/j.ejogrb.2010.12.030
Alatas C, Aksoy E, Akarsu C, Yakin K, Bahceci M (1996) Hemodynamic assessment in pelvic inflammatory disease by transvaginal color Doppler ultrasonography. Eur J Obstet Gynecol Reprod Biol 70(1):75–78 pii: S0301211596025432
Tepper R, Aviram R, Cohen N, Cohen I, Holtzinger M, Beyth Y (1998) Doppler flow characteristics in patients with pelvic inflammatory disease: responders versus nonresponders to therapy. J Clin Ultrasound 26(5):247–249
Davis JA, Gosink BB (1986) Fluid in the female pelvis: cyclic patterns. J Ultrasound Med 5(2):75–79
Molander P, Cacciatore B, Sjoberg J, Paavonen J (2000) Laparoscopic management of suspected acute pelvic inflammatory disease. J Am Assoc Gynecol Laparosc 7(1):107–110
Acknowledgments
We thank Dr. Povilas Sladkevicius for providing us with ultrasound images.
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romosan, G., Valentin, L. The sensitivity and specificity of transvaginal ultrasound with regard to acute pelvic inflammatory disease: a review of the literature. Arch Gynecol Obstet 289, 705–714 (2014). https://doi.org/10.1007/s00404-013-3091-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-3091-6