Abstract
Background
Ovarian cancer (OC) is the leading cause of death in women with gynecological cancer. Most patients are diagnosed at advanced stage with poor prognosis. Currently, surgical tumor debulking followed by chemotherapy based on platinum and taxane is the standard treatment for advanced OC. However, these patients remain at great risk for recurrence and developing drug resistance. Therefore, new treatment strategies are needed to improve outcomes for patients with advanced and recurrent OC. Olaparib (AZD2281, KU-0059436), as one of the best understood Poly-(ADP-ribose) polymerase (PARP) inhibitor targeting DNA repair mechanisms, caused more and more attention. Clinical trial data of Olaparib had been cumulated, which applied as the single-agent in relapsed OC monotherapy, especially for BRCA mutation associated OC.
Methods
In this review, we demonstrated the mechanism of PARP inhibitors and summarized clinical trial data and clinical development of Olaparib targeted OC in order to address a new promising therapy strategy for advanced relapsed OC.
Conclusion
Given the unprecedented clinical potential of Olaparib, the further research on Olaparib will have great significance in selection of OC patient populations that will respond to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is the fifth leading cause of death from cancer among women and the most lethal gynecologic malignancy [1]. Although the combination of radical surgery with platinum-taxane-based chemotherapy is initially effective, most patients experience relapse. In the last decades, some important advances have been made in both surgical [2] and anticancer strategies, but only modest improvements in outcome have been reached. Therefore, new treatment approaches are required and recently, target therapies have gained great attention. These agents, who interfere solely with specific molecular targets, hold the promise of greater selectivity and lower toxicities than traditional cytotoxic drugs.
In this scenario, the role of repair pathways of DNA has emerged as a further critical target, being a fundamental step for maintenance of genome integrity and for the response to DNA-damaging chemotherapy. Poly (ADP-ribose) polymerase (PARP) plays a critical role in the repair of DNA single-strand breaks (SSB) through the base excision repair (BER) pathway. PARP inhibitors designed to target DNA repair pathways and targeting BER pathway appears one of the most promising in OC.
Up to now, the most investigated PARP inhibitor is Olaparib (AZD2281, KU-0059436). As an important novel agent of anticancer drug, there had been several clinical trials in development with Olaparib in the treatment of cancer. However, a comprehensive understanding of the therapeutic applications of Olaparib in tumor cells was not available until recently. Within this review, we briefly summarize the recent research progress on the efficacy and tolerability of Olaparib in OC patients with or without BRCA mutation. Hopefully the information in this article summarizing the recent studies advances will lead to a better understand of the mechanisms of Olaparib in genome stability maintenance and provide valuable clues in the selection of patient populations that will respond to Olaparib.
PARP and its inhibitor
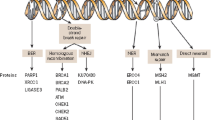
PARP, is an enzyme involved in BER, has been known since 1963 when Chambon et al. [3] wrote “Nicotinamide Mononucleotide Activation of New DNA-Dependent Polyadenylic Acid Synthesizing Nuclear Enzyme”. The PARP family consists of 17 proteins if based on structural similarity. PARP1 is the protein that is best understood and the first reported PAR polymerase in SSB repair, which plays important roles in multiple DNA damage response pathways (Fig. 1a). It includes a nuclear localization signal and is an abundant nuclear protein. PARP1 mainly contains three functional domains: the N-terminal DNA-binding domain (DBD), the central auto-modification domain (AMD), and the C-terminal catalytic domain (CD). The DBD includes three zinc finger motifs. The first two (Zn I and Zn II) participate in the recognition of double strands break/SSB and mediate the binding of PARP1 to DNA. The newly identified third zinc finger motif (Zn III) mediates the regulation of the DBD on the catalytic activity and is not believed to be involved in DNA-binding [4, 5]. The AMD contains specific glutamate and lysine residues serving as acceptors for ADP-ribose moieties and also a BRCT domain that can interact with many DNA damage response proteins. The CD includes a PARP signature motif and a WGR motif and catalyzes the formation of PAR. The PARP signature motif forms the active site and binds nicotinamide adenine dinucleotide (NAD+). The function of the WGR motif is unknown [6–8].
a Structure of PARP1. b Function of PARP inhibitors. ① Many commonly utilized cancer chemotherapy regimens target tumor cells via DNA single strand break (SSB). ② Key DNA repair pathways (such as: PARP) are up-regulated in tumor cell may lead to resistance to chemotherapy. ③ Inhibiting PARP may potentiate chemotherapy or be used as monotherapy
In 1971, nicotinamide was found to be a weak inhibitor of PARP [9]. The first generation of inhibitors included nicotinamide analogs. The first widely tested agent, 3-aminobenzamide, developed in 1980 [10], was not as selective and 1,000 times less potent compared with newer inhibitors. The second generation, including PD128763, NU1025, was 50 times more potent than 3-aminobenzamide. Current PARP inhibitors in development are the third-generation PARP inhibitors and have greater potency and specificity for PARP [11] (Table 1). Importantly, Olaparib is one of the best understood third-generation PARP inhibitors. Moreover, compelling data indicate that PARP1 inhibitors result in synthetic lethality in homologous recombination (HR)-deficient tumor cells. The rationale for PARP1 inhibitors is that by inhibiting BER, these agents can prevent repair that occurs after cytotoxic chemotherapy that causes SSB (Fig. 1b). PARP1 inhibitors compete with NAD+ at the enzyme’s active site. Because this site is present in other enzymes, PARP inhibitors might act in a non-specific manner.
Characteristics of Olaparib
Chemistry
Olaparib (4-[(3-[(4-cyclopropylcabonyl)piperazin-4-yl]carbonyl)-4 fluorophenyl] methyl(2H) phthalazin-1-one) is an oral PARP inhibitor with IC50 of 4.9 nM for PARP1, which appeared as an off-white to pale yellow/pale orange crystalline solid, with melting point 210–211 °C and has a molecular weight of 434.47 Da [12] (Fig. 2).
Pharmacodynamics
The effective concentration for inhibiting cellular PARP1 activity by >90 % is approximately 30–100 Nm of Olaparib in several tumor cell lines including ovarian A2780, breast MCF-7 and colorectal SW620. The media effective concentration (required to induce a 50 % effect [EC50]) for Olaparib is approximately 6 M. This was assayed by measuring inhibition of poly (ADP-ribose) (PAR) chain formation in SW620 cellular lysates following incubation of Olaparib (0.1–300 nM). Concentration in the active range of between 0.2 and 4 μM, with a mean of 1 μM are known to be achievable within tumors in vivo [12].
Metabolism
In caner patients, following single capsule oral dose, Olaparib was rapidly absorbed. Mean apparent volume of distribution was 40.3 L, mean apparent plasma clearance was 4.55 L/h and the mean terminal half-life (t 1/2) was 6.10 h. Exposure increased proportionally with dose at doses up to 100 mg bd (twice daily) but increased in a less than proportional fashion at higher doses. Following dosing to cancer patients at doses of 400 mg bd, the population estimated maximum plasma concentration at steady state (C max ss) ranged from 1.45 to 11.0 μg/mL (3.34–25.3 μM, equivalent to unbound concentrations of 0.26–1.99 μg/mL [0.6–4.58 μM]); the steady-state area under the plasma concentration–time curve (AUC) (area under plasma concentration–time curve from zero to 12 h [AUC 0–12]) ranged from 6.56 to 122 μg h/mL. Following administration of a radiolabeled dose of Olaparib to cancer patients, Olaparib accounted for approximately 70 % of the circulating material in the plasma with the remainder accounted for by three other components (each accounting for approximately 10 % of the material). Drug-related material was eliminated in the urine (35–50 %) and in the feces (12–60 %) with 10–20 and 0.6–14 % of the dosed material recovered in the urine and feces as unchanged drug, respectively [13].
Safety and tolerability
Olaparib monotherapy appears to be generally well tolerated across studies at doses up to and including the maximum tolerated dose (MTD) of 400 mg bd. Olaparib monotherapy at 400 mg bd was generally well tolerated in patients with platinum-sensitive serous OC [14] and BRCA OC [15]. Preliminary data from dose escalation studies of Olaparib in combination with various chemotherapy agents indicate an increase in bone marrow toxicity (anemia, neutropenia, thrombocytopenia) greater than expected if the agents had been administered alone. Administration of Olaparib in combination with Dacarbazine, Topotecan, or Paclitaxel resulted in a lower MTD compared with administration as a monotherapy. Administration of Bevacizumab at recommended doses with Olaparib up to 400 mg bd was a well-tolerated combination treatment resulting in no unusual or unexpected adverse events (AEs).
Hematological toxicities associated with Olaparib include anemia and lymphopenia and are usually manageable. Interestingly, the most frequently documented drug-related toxicities are gastrointestinal symptoms (nausea, vomiting) and fatigue, which may occur in up than 20 % of patients and may present of moderate/severe entity, more frequently than hematological AEs [13, 16]. In addition, in a small number of patients, pneumonitis or myelodysplastic syndrome/acute myeloid leukemia have been observed [13, 15–18].
Major clinical trials of Olaparib as single-agent therapy
As shown in Table 2, in OC, six primary papers have been published concerning the clinical studies of Olaparib, including two phase I studies and four phase II studies.
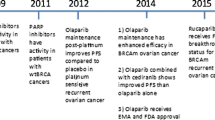
In 2009, considering cell lines lacking wild-type BRCA1/2 are highly sensitive to PARP inhibitors compared to heterozygous mutant or wild-type cells [19], Fong et al. [13] conducted the Phase I trial with dose escalation of Olaparib from 10 mg daily to 600 mg bd in a population enriched in carriers of a BRCA1/2 mutations. The study included 60 patients with histologically or cytologically confirmed refractory solid tumors, including 21 OC patients. Interestingly, inhibition of PARP by more than 90 % was observed in cells from patients treated with 60 mg or more of Olaparib bd, measured in peripheral mononuclear cells. Dose-limiting toxicities were observed at 400 and 600 mg bd; the maximum MTD was 400 mg bd. AEs were minimal (predominant gastrointestinal and fatigue). Clinical benefit, with either radiological or tumor-marker response or stable disease for a period of 4 months or more, was achieved in 63 % of patients with BRCA-related cancer, including eight OC patients. The important finding of this study was the observation of objective antitumor activity in platinum resistant patients at dosage well below the recommended MTD [13, 20].
These promising data of the above trial were lately confirmed by the same team in an expanded cohort of 50 patients with advanced BRCA1/2 mutation-associated ovarian, primary peritoneal, and fallopian tube cancers (13 platinum sensitive, 24 platinum resistant, and 13 platinum refractory) [21]. The clinical benefit rate was 46 % in the expansion cohort, with median response duration of 28 weeks (range 10–86 weeks). The overall clinical benefit rate decreased significantly with platinum insensitivity (platinum-sensitivity: 69 %, platinum-resistant: 46 %, platinum-refractory: 23 %). Furthermore, there was a positive association between the overall platinum-free interval and response to Olaparib (P = 0.002). Consistent with previously reported AEs in the dose-escalation cohorts, the most common drug-related toxicities in this study were gastrointestinal symptoms and fatigue. Their findings suggest that Olaparib correlate with platinum sensitivity in addition to showing a benefit in resistant and refractory patients.
The promising data of Olaparib reported by Fong et al. [13, 21] was pursued rapidly into Phase II clinical trials with preselected patient group of advanced OC. Audeh et al. showed that greater Olaparib activity is seen at high dose of 400 mg bd than 100 mg bd (objective response rate: 33 vs. 13 %, media of PFS: 5.8 vs. 1.9 months, P < 0.05) in the Phase II international multicentre single arm, open label sequential dosing cohort study of BRCA1/2 mutation carriers with recurrent EOC [18]. Two patients in the 400 mg cohort had complete responses and none in the lower dose group. Toxicity was mild with severe AE of grade 3–4 infrequent.
Upon the indication of a dose–response relationship and in order to address the role of Olaparib as a second-line treatment in BRCA1/2 mutated patients with OC, a three-arm study comparing two different dosages of Olaparib with the reference was planned. Kaye et al. demonstrated that 97 patients with BRCA-mutated progressive or recurrent disease <12 months after their last platinum were randomized in a 1:1:1 ratio to receive Olaparib 200 mg or 400 mg bd or pegylated liposomal doxorubicin (PLD) 50 mg/m2 intravenously [15]. Objective response rates were 25, 31, and 18 % for Olaparib 200 mg, Olaparib 400 mg, and PLD, respectively, statistically comparable across the three arms. No statistically significant differences in PFS (200 mg: 6.5 months vs. 400 mg: 8.8 months vs. PLD: 7.1 months; P = 0.6) have been observed. Remarkably, the median PFS of 7.1 months of PLD observed in this trial is considerably greater than the 4 months expected, taking as a reference the large randomized trial from Gordon et al. [22], in a similar mix of platinum-resistant and sensitive cancers and which definitively defined PLD as standard treatment for recurrent OC. These results are also in accordance with the retrospective data published by Adams et al. [23] showing a higher activity of PLD in BRCA-mutated OC patients. Is therefore possible to speculate that that HR deficient OC may have a better clinical outcome from anthracyclines such as PLD compared to unselected cases? That warrants further study.
As mentioned above, one of the greatest questions to be answered is whether or not the activity of Olaparib, in general, is limited to tumors with BRCA mutations or is also directed throughout those tumors having the property of ‘BRCAness’. Gelmon et al. recently addressed this issue in a Phase II single-arm study of Olaparib in patients with high-grade serous/undifferentiated OC with unknown BRCA status or BRCA-negative. In addition, there was a reference group known to have germ-line BRCA mutations treated within the trial [16]. Indeed, 90 patients were enrolled (64 OC and 26 breast cancer) and treated with Olaparib 400 mg bd. After BRCA testing, 17 patients showed BRCA mutations. Consistently with previous studies, objective responses were seen in 41 % patients with BRCA1 or BRCA2 mutations and 24 % without mutations. The most common AEs were gastrointestinal and fatigue, which analogously with previous studies. This study represents the first important evidence of PARP inhibitor activity in non-BRCA mutant OC. Similar data for relapsed OC, irrespective for BRCA mutations, have been recently found in a randomized, double-blind, placebo-controlled, Phase II study by Ledermann et al. [14]. Their trial involved patients with platinum-sensitive, relapsed, high-grade serous OC who had received two or more platinum-based regimens and had a partial or complete response to their most recent platinum-based regimen with the aim to evaluate the efficacy of Olaparib monotherapy as maintenance treatment. Of 265 randomized patients, 136 received Olaparib and 129 received placebo. Median PFS was 8.4 months for the study group, compared with 4.8 months of the control group (P < 0.001). AEs more commonly reported in the Olaparib group than in the placebo group but the majorities of AEs were grade 1–2. Nevertheless, no significant difference between groups of overall survival (P = 0.75) in interim analysis. This data should be interpreted with caution, considering that at the moment of publication of the manuscript, 21 % of the patients were still receiving Olaparib (and 3 % were still receiving placebo), which indicates that the disease is controlled for a prolonged period in some patients.
Additionally, on December 22, 2011, a financial new sentences that Astra Zeneca canceled Olaparib Phase III trials. The drug-maker said that at this moment its investigational compound would not progress into Phase III development for the maintenance treatment of serous OC, because significant PFS observed in the study from Ledermann et al. [14] did not translate into an overall survival benefit, even if data were still not mature [24].
Olaparib in combined therapies
However, what are the effects of Olaparib with other drugs, either cytotoxic agents or target therapies finalized to other molecular pathways?
The rationale for association with chemotherapy is based on two potential mechanisms: the enhancement of chemosensitivity by blocking BER, which may be responsible of drug resistance and the potential increase in cell death and activity of PARP inhibitor through tumor-selective synthetic lethality, brought about by the additional exogenous DNA damage caused by chemotherapy [25–27]. Up to now, association of Olaparib and cytotoxic agents such as Topotecan [28], Dacarbazine [29], Paclitaxel [30], and Cisplatin plus Gemcitabine [31] have been described in four clinical trials of Phase I. In all these studies, an overlapping, dose-limiting toxicity, particularly myelosuppression, has emerged, more pronounced than that seen with chemotherapeutic agents alone, which maybe attribute to an increase in Olaparib exposure in the presence of cytotoxic agents, which causes DNA double-strand breaks, could potentially explain the higher degree of myelosuppression due to an effect on rapidly dividing bone marrow cells.
Therefore, in the beginning of current year, the safety and tolerability of Olaparib have also been tested in another Phase I trial evaluating the association regimen with Bevacizumab in patients affected by advanced solid tumors [32]. In the study from Dean et al. increasing doses of continuous oral Olaparib (100, 200, and 400 mg) in combination with endovenous Bevacizumab (10 mg/kg every 2 weeks) have been administrated. The evidence that the vessel regression caused by the anti-angiogenic agent induces hypoxia and results in an increase of DNA damage and genetic instability [33] represents the basis for this association. Combining Olaparib and Bevacizumab is based on the rationale that direct targeting of PARP by Olaparib and indirect sensitization to Olaparib by acquisition of HR defects by Bevacizumab will be therapeutically beneficial.
Conclusions
In summary, the Phase I/II clinical data of Olaparib we reviewed in the treatment of advanced OC and Olaparib has shown great clinical promise, particularly as it represents a real departure from conventional chemotherapy. Given the unprecedented clinical potential of Olaparib, the further research on Olaparib will have great significance, not only in elucidation of the complex mechanisms of Olaparib with respect to DNA repair, but also in selection of patient populations that will respond to treatment.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C, Barakat RR (2009) Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol 114(1):26–31
Chambon P, Weill JD, Mandel P (1963) Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun 11:39–43
Langelier MF, Servent KM, Rogers EE, Pascal JM (2008) A third zinc-binding domain of human poly(ADP-ribose) polymerase-1 coordinates DNA-dependent enzyme activation. J Biol Chem 283(7):4105–4114
Tao Z, Gao P, Hoffman DW, Liu HW (2008) Domain C of human poly(ADP-ribose) polymerase-1 is important for enzyme activity and contains a novel zinc-ribbon motif. Biochemistry 47(21):5804–5813
Hassa PO, Haenni SS, Elser M, Hottiger MO (2006) Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev 70(3):789–829
de Murcia G, Schreiber V, Molinete M, Saulier B, Poch O, Masson M, Niedergang C (1994) Ménissier de Murcia J. Structure and function of poly(ADP-ribose) polymerase. Mol Cell Biochem 138(1–2):15–24
Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG (2010) PARP inhibition: PARP1 and beyond. Nat Rev Cancer 10(4):293–301
Clark JB, Ferris GM, Pinder S (1971) Inhibition of nuclear NAD nucleosidase and poly ADP-ribose polymerase activity from rat liver by nicotinamide and 5′-methyl nicotinamide. Biochim Biophys Acta 238(1):82–85
Durkacz BW, Omidiji O, Gray DA, Shall S (1980) (ADP-ribose)n participates in DNA excision repair. Nature 283(5747):593–596
Weil MK, Chen AP (2011) PARP inhibitor treatment in ovarian and breast cancer. Curr Probl Cancer 35(1):7–50
Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A et al (2008) 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem 51(20):6581–6591
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M et al (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361(2):123–134
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G et al (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366(15):1382–1392
Kaye SB, Lubinski J, Matulonis U et al (2012) Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 30(4):372–379
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K et al (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12(9):852–861
Yamamoto N, Nokihara H, Yamada Y et al (2012) A phase I, dose-finding and pharmacokinetic study of Olaparib (AZD2281) in Japanese patients with advanced solid tumours. Cancer Sci 103(3):504–509
Audeh MW, Carmichael J, Penson RT et al (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376(9737):245–251
Ashworth A (2008) A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26(22):3785–3790
Underhill C, Toulmonde M, Bonnefoi H (2011) A review of PARP inhibitors: from bench to bedside. Ann Oncol 22(2):268–279
Fong PC, Yap TA, Boss DS et al (2010) Poly (ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 28:2512–2519
Gordon AN, Fleagle JT, Guthrie D et al (2001) Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19(14):3312–3322
Adams SF, Marsh EB, Elmasri W et al (2011) A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol Oncol 123(3):486–491
Marchetti C, Imperiale L, Gasparri ML, Palaia I, Pignata S, Boni T, Bellati F (2012) Benedetti Panici P. Olaparib, PARP1 inhibitor in ovarian cancer. Expert Opin Investig Drugs 21(10):1575–1584
Palma JP, Rodriguez LE, Bontcheva-Diaz VD et al (2008) The PARP inhibitor, ABT-888 potentiates temozolomide: correlation with drug levels and reduction in PARP activity in vivo. Anticancer Res 28(5A):2625–2635
Donawho CK, Luo Y, Luo Y et al (2007) ABT-888, an orally active poly (ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumour models. Clin Cancer Res 13(9):2728–2737
Carden CP, Yap TA, Kaye SB (2010) PARP inhibition: targeting the Achilles’ heel of DNA repair to treat germline and sporadic ovarian cancers. Curr Opin Oncol 22(5):473–480
Samol J, Ranson M, Scott E et al (2011) Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, Olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumours: a phase I study. Invest New Drugs 30(4):1493–1500
Khan OA, Gore M, Lorigan P et al (2011) A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer 104:750–755
Dent RA, Lindeman GJ, Clemons M et al (2011) Safety and efficacy of the oral PARP inhibitor Olaparib (AZD2281) in combination with paclitaxel for the first or second-line treatment of patients with metastatic triple-negative breast cancer: results from the safety cohort of a phase I/II multicenter trial. J Clin Oncol 28(15S):1018 (abstract)
Rajan A, Carter CA, Kelly RJ et al (2012) A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumours. Clin Cancer Res 18(8):2344–2351
Dean E, Middleton MR, Pwint T et al (2012) Phase I study to assess the safety and tolerability of Olaparib in combination with Bevacizumab in patients with advanced solid tumours. Br J Cancer 106(3):468–474
Chan N, Bristow RG (2010) Contextual synthetic lethality and/or loss of heterozygosity: tumour hypoxia and modification of DNA repair. Clin Cancer Res 16:4553–4560
Acknowledgments
This work was supported by Tianjin Health Bureau of Science and Technology Funds (2012KZ073).
Conflict of interest
None of the authors has any potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Chen and L. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, L. & Hao, Q. Olaparib: a promising PARP inhibitor in ovarian cancer therapy. Arch Gynecol Obstet 288, 367–374 (2013). https://doi.org/10.1007/s00404-013-2856-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2856-2