Abstract
Purpose
To investigate the association between C421T polymorphism within exon 4, C575T polymorphism within exon 6 of the RANK gene and bone mineral density (BMD) variations in postmenopausal Turkish women.
Methods
One hundred seventy-eight postmenopausal women (patients = 100 and controls = 78) who applied to Ege University Faculty of Medicine, Department of Physical Medicine and Rehabilitation, for osteoporosis examination were analyzed. BMDs of the lumbar spine and femoral sites were measured. Patient and control groups were established based on their T-score values being above and/or below −1. After venous blood sampling, C421T and C575T polymorphisms of the RANK gene were assessed through PCR process following DNA extraction.
Results
Genotype frequencies for the C421T and C575T polymorphisms were compared between the control group and the patient group. No significant difference was detected between the two groups for both polymorphisms. There was also no significant difference between the control and patient groups in terms of the combined genotype (p = 0.752) and the combined haplotype analysis of the C421T and C575T polymorphisms (p = 0.723). In the control and patient groups separately, no significant differences in BMD values either at the femoral sites or at the lumbar spine were detected between the combined genotypes of the two polymorphisms.
Conclusions
The genotypes, combined genotypes and allele frequencies of C421T and C575T polymorphisms of the RANK gene have not been found to be associated with BMD in Turkish women. Further studies including both sexes and more cases are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a chronic, progressive and multifactorial disease of the bone metabolism, characterized by increase in bone fragility and risk of fracture resulting from a decrease in bone strength [1].
Osteoporosis, under the influence of multiple genetic, physical, hormonal and nutrition-related pathogenetic factors, either alone or combined, leads to a loss in bone mass and disruption in bone micro-architecture [2, 3]. It has been estimated that genetic factors account for up to 70 % of individual variance in bone mineral density [4].
Due to the fact that osteoporosis is a polygenic disease, studies comparing normal and osteoporotic individuals must be carried out in order to identify the possible roles of specific genes in the pathogenesis of osteoporosis. This has recently been demonstrated through estrogen receptor polymorphisms [5].
Many studies tried to find the candidate gene for osteoporosis with the conflicting data in various populations and the results are usually controversial. Til date, only a few genes that are strongly relevant to BMD and risk of fracture have been identified. Five epidemiologic cohort studies were examined for 150 candidate genes which might be relevant to BMD and risk of fracture in 19,195 adult Europeans 14,277 of whom were females in a meta-analysis by Richard et al. [6]. It was reported that single nucleotide polymorphisms (SNPs) in several genes have not showed a significant relationship with BMD and risk of fracture. In the same meta-analysis, 241 SNPs, which have been found in nine genes [lipoprotein receptor-related protein-4 (LRP4), LRP5, tumor necrosis factor superfamily-11 (TNFSF11) or receptor activator of nuclear factor kappa B ligand (RANKL), tumor necrosis factor receptor superfamily-11A (TNFRSF11A = RANK), TNFRSF11B (OPG = osteoprotegerin), secreted phosphoprotein-1(SPP1) (OPN = osteopontin), integrin alpha-1 (ITGA1), SOST gene (gene product sclerostin) and estrogen receptor-1 (ESR1)] have been reported to be associated with BMD at the femoral neck and/or lumbar regions.
Recently, it has been discovered that NF-κB (RANK) and RANK ligand (RANKL) system play an important role in the bone remodeling. It has been reported that the activating mutation of exon 1 of the RANK gene results in an increase of RANK-mediated nuclear factor kappa B (NF-κB) signaling, osteoclast formation and osteoclastic activity [7]. The association of the RANK gene polymorphisms and BMD has been examined in various studies, and varying results depending on sex, ethnic origin and differences in the bone regions have been reported [8]. If we consider the genes responsible for the bone remodeling, RANK comes forward among all and the two polymorphisms (C421T and C575T) in RANK gene were the two strong candidates for which the results obtained have been inconsistent and depend on the ethnicity of study participants [10–12]. Therefore, we decided to investigate the possible association between the suggested polymorphisms of the RANK gene and bone mineral density in postmenopausal Turkish women. Besides, there has been no study reported in the Turkish population with these polymorphisms up to date. Therefore, we aimed to investigate the association between C421T (rs35211496) and C575T (rs1805034) RANK gene polymorphisms and BMD variations at the femoral and lumbar regions (which has not been reported in Turkish population) in postmenopausal Turkish women aged between 45 and 65 years.
Materials and methods
Study population
Postmenopausal women (n = 178) aged between 45 and 65 years who applied to Ege University Faculty of Medicine, Department of Physical Medicine and Rehabilitation and Department of Gynaecology and Obstetrics outpatient clinics for osteoporosis between January 2008 and April 2010 were enrolled to the study. Cases having normal BMD values were included in the control group (n = 78) and cases having low BMD values in the patient group (n = 100). BMD was considered as “low” if T-score at the lumbar spine and/or femoral sites (femur neck and total) was below −1; and “normal” if T-scores at the femoral and lumbar regions were −1 and/or higher. The study was approved by the Research Ethics Committee of Ege University Faculty of Medicine. Written informed consent was obtained from all the participants before enrollment into the study. To determine the homogeneity of the patient and the control groups, all of the participants were subjected to the same questionnaire including the factors which have been possibly considered to be affecting BMD. All the volunteers were questioned in terms of weight, height, body-mass index (BMI), menarche and menopause age, duration of reproductive time period, cumulative pregnancy period in reproductive period, breast-feeding duration, physical activity score and alcohol and nicotine consumption (pack-year). The daily amount of alcohol intake was calculated as gram/day using the DIARI, an individual records type and volume (in liter) of drinks in the categories of beer, wine, Turkish raki and others. BMD values and T-score values at the femoral and lumbar regions of the cases were also recorded. Menopause was identified as the amenorrhea for at least 12 consecutive months and with FSL levels >40 IU/L and confirmed by detailed history and exam [9].
Cases having a history of any bone disease or autoimmune disease, endocrine or metabolic disease which can affect bone metabolism and history of treatment relevant to these diseases (thyroid or parathyroid dysfunction, Cushing’s disease, Addison’s disease, acromegaly, Paget’s disease, osteomalacia, type 1 or type 2 diabetes mellitus), history of inflammatory rheumatologic pathology, neurologic disease which can lead to low BMD or history of immobilization for more than 3 months, history of treatment which can affect BMD (bisphosphonate, strontium ranelate, calcium and vitamin D, teriparatide, etc.), exposure to oral/inhaler/intravenous glucocorticoid, malabsorption, cardiac or liver failure, renal disease, history of anticoagulant treatment, malignancy, history of anticonvulsant drug use, hormone replacement therapy, past/current chronic alcoholism, surgical menopause, menopause before the age of 40 were excluded from the study.
Body-mass index (BMI) was calculated for all the cases included in the study by the formula of dividing body weight (kg) by the square of height (m2). BMD values at the lumbar spine (L1–L4 vertebra), and femoral sites (femur total and femoral neck) were measured in g/cm2 using Hologic QDR 4500 dual energy X-ray absorptiometry machine (Hologic QDR Delphi-A, Bedford, MA 01730, USA). T-scores for the same regions were also recorded. The coefficient variation of this technique using the Hologic QDR 4500 densitometer is 1.0 % for the lumbar spine and 1.0 % for the total femur in our clinic.
DNA extraction
Venous blood was drawn into 2 ml collection tubes with EDTA from patients (n = 78) and controls (n = 100). DNA extraction was performed with Magna Pure LC (Roche Molecular Biochemicals, Mannheim, Germany) kit according to the established protocol. Success of DNA extraction was confirmed by 2 % agarose gel.
Detection of RANK gene C421T polymorphism
In order to detect the RANK gene C421T polymorphism within exon 4; PCR was performed using Forward: 5′tctgggctggatgttggatagc3′ and Reverse: 5′ tagcaccctggagctttcttgc3′ primers. PCR reaction mixture consisted 12.2 μl distilled water, 2.5 μl 10× PCR buffer, 2 μl MgCl2 (25 mM), 1 μl dNTP (deoxynucleotide triphosphate) (4 × 25 μmol), primers (1 μl each) and 0.3 μl Taq polymerase (Fermentas, Thermo Scientific, Waltham, MA, USA). PCR conditions were set as: 95 °C–3 min, 33 cycles of 95 °C–40 s/56 °C–40 s/72 °C–45 s, 72 °C–10 min. After amplification, purification was carried out in order to eliminate excess PCR components (Fermentas Genejet PCR Purification Kit, Thermo Scientific, Waltham, MA, USA). Subsequent to purification, chain termination method was performed using Big Dye Terminator kit (Applied Biosystems, Foster City, CA, USA) according to the cycle sequencing protocol with standard techniques. Another purification process was carried out in order to remove the excess fluorescent-marked dideoxy nucleoside triphosphates (ddNTPs) PCR components emerging after the sequence process (Zymo Research DNA Sequencing Clean Up Kit, Zymo Research Inc, Orange, CA, USA). After the purification, samples were conducted on the ABI 310 (Applied Biosystems, Foster City, CA, USA) sequencer in line with the sequence protocol. Each sample was analyzed using Gene-Mapper software (Applied Biosystems, Foster City, CA, USA). Genotyping for RANK gene in exon 4 was performed using Chromas available at http://www.ncbi.nlm.nih.gov.
Detection of RANK gene C575T polymorphism
In order to detect the RANK gene C575T polymorphism within exon 6; PCR was performed using Forward: 5′ggggattcaaatgtccaagaagg3′ and Reverse: 5′catgcacgggatgaaataaaggg3′ primers. PCR reaction mixture was the same as the one mentioned above for C421T. PCR conditions were set as; 95 °C–3 min, 33 cycles of 95 °C–40 s/56 °C–40 s/72 °C–45 s, 72 °C–10 min. After amplification, PCR–RFLP method was used for genotyping. Briefly, a mixture of 10 μl of PCR product, 2 μl of buffer, and 1 μl of SsiI enzyme was incubated for 2 h at 37 °C.
Digested PCR products were visualized by 3.5 % agarose gel. Uncut PCR product was identified as 353 bp. In case of CC genotype, 188- 130- 36-bp bands would be obtained. In CT genotype, 316- 188- 130- 36-bp products and in TT genotype 316- 37-bp products were expected.
Statistical analysis
The prevalence of osteoporosis in women between the ages of 50 and 60 was assumed to be in the range of 40–55 %. Sample size estimations with the computer software PASS (version 6.0) indicated that 256 patients were needed to attain 80 % statistical power, assuming an alpha of 0.05. Lack of funding and a short time period gave rise to a power under 80 % in the present study.
Variables that conform to normal distribution (e.g., age, height, BMD values) were given as mean and standard deviation (mean ± SD); continuous variables that do not conform to normal distribution (e.g., breast-feeding duration, physical activity score) were given as median and interquartile range (median ± IR). Conformity to normal distribution in numeric variables was tested with Shapiro–Wilk statistical analysis. For comparing two groups, Student t test or Mann–Whitney U test was selected.
For the classified variables (genotype and allele frequencies), cross-tabulations in the patient–control groups were created and Chi-square (χ 2) statistical analysis was conducted.
In the patient–control group, logistic regression analyses were conducted and odds ratios (OR) were calculated in order to determine the risk ratios of the combined genotypes.
Generalized linear model (GLM) was used to check the significance between polymorphisms variance after eliminating the influence of the variables of age, weight, height and menopause age which were considered to have an influence on variations in BMD values in the comparison of arithmetical means of BMD for the regions examined in the patient and control groups for C421T and C575T polymorphisms.
Threshold level for statistical significance was established as 0.05 (p < 0.05). SPSS 18.0 software was employed for all the analyses.
HelixTree Version 5.0.0 genetic analysis software was employed for the analysis of the Hardy–Weinberg equilibrium, combined haplotype frequencies and significance of the control group.
Results
Statistics of the demographic data and descriptive data
Basic descriptive statistics of the patient and control groups are given in Table 1. Weight, BMI and alcohol consumption were significantly higher in the control group.
Comparison of genotype distributions and allele frequencies between the control and patient groups
The genotype distribution of both polymorphisms in the control group was in Hardy–Weinberg equilibrium (p > 0.05).
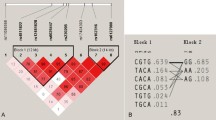
When the control and patient groups were examined together, it was detected that the two polymorphisms were inherited together (p = 0.023).
For RANK C421T polymorphism within the patient group (n = 100), CC genotype was detected in 67 (67 %), CT genotype in 30 (30 %) and TT genotype in 3 cases (3 %). Within the control group (n = 78), CC genotype was detected in 56 (71.8 %), CT genotype in 20 (25.6 %) and TT genotype in 2 cases (2.6 %).
When genotypes for the C421T polymorphism were compared between the control and patient groups, no significant difference was detected between the two groups (χ 2 = 0.472, p = 0.790). When the C and T allele frequencies for the C421T polymorphism in the control and patient groups were calculated, the T allele was found to be 1.2 times higher in patient group with respect to the control, which was not found to be significant (χ 2 = 0.428, p = 0.512).
For RANK C575T polymorphism within the patient group (n = 100), CC genotype was detected in 32 (32 %), CT genotype in 35 (35 %) and TT genotype in 33 cases (33 %). Within the control group (n = 78), CC genotype was detected in 29 (37.2 %), CT genotype in 30 (37.5 %) and TT genotype in 19 cases (24.4 %).
In the comparison of genotypes for C575T polymorphism between the control and patient groups, no difference was detected between the two groups (χ 2 = 1.607, p = 0.448). A difference in terms of the C and T allele frequencies for the C575T polymorphism was not detected between the control and patient groups (χ 2 = 1.678; p = 0.195)
Comparison of combined genotype distributions between the control and patient groups
It was detected that there were no significant differences in the combined genotype distribution of the C421T and C575T polymorphisms between the control and patient groups (χ 2 = 4.235, p = 0.752). When the combined genotypes were calculated separately, no significant difference between the control and patient groups was observed either. With respect to the CCRANK4/CCRANK6 combined genotype, it was detected that the risk of presence of the CTRANK4/TTRANK6 combined genotype was 2.3 times higher in the patient group, which was not considered to be significant (p = 0.195). A statistically significant combined genotype pattern was not revealed in the combined genotype analyses of the C421T and C575T polymorphisms for the control and patient groups.
Combined haplotype analysis in the control and patient groups
No significant difference between the control and patient groups was detected in the combined haplotype analysis (χ 2 = 1.324, p = 0.723). A statistically significant combined haplotype pattern was not revealed in the combined haplotype analyses of the C421T and C575T polymorphisms.
Adjusted BMD values of the control and patient groups with respect to genotypes
The arithmetical means of BMD values with respect to the genotypes for each of the C421T and C575T polymorphisms in the control and patient groups were recalculated by correcting the age, height, weight and years since menopause.
BMD values of the patient and control groups corrected by genotypes for the C421T polymorphism were calculated after combining the homozygote mutant and heterozygote groups, polymorphism which would create a significant difference in the BMD values for all the three zones were not encountered within the control and patient groups (Table 2).
No significant difference was observed in the BMD values of all the three regions for the C575T polymorphism between the CC, CT, TT genotypes in the control and patient groups (Table 2).
Adjusted BMD values of the control and patient groups with respect to combined genotypes
As there were only two cases having the TT/TT combined genotype in each one of the control and patient groups, a new analysis was conducted for the control and patient groups by forming a group of all the combined genotypes except the (CC/CC) combined genotype. No significant difference in any of the adjusted BMD values between the CC/CC combined genotype group and the other combined-genotypes group was detected within the control (p FN BMD = 0.526, p FT BMD = 0.757, p L1–4 BMD = 0.778) and the patient group (p FN BMD = 0.363, p FT BMD = 0.307, p L1–4 BMD = 0.399) (Table 3).
Both RANK C575T polymorphism and RANK C421T polymorphism were not found as risk factors associated with low BMD for single genotype (OR, 0.85; 95 % CI 0.41–1.73 and OR, 0.86; 95 % CI 0.41–1.80, respectively).
Discussion
The association between the RANK gene polymorphisms and osteoporosis has been discussed in the literature [10–15]. 15 out of the 18 SNPs studied in two of these trials were in the protein non-coding intronic part, the remaining 3 were SNPs located in the 3′-UTR (untranslated region) zone [14, 15]. Both polymorphisms that we have studied in our trial have been located in the protein coding (exon) part. In the RANK protein, depending on the RANK gene C421T polymorphism within exon 4, tyrosine amino acid was replaced by histidine (Hys141Tyr) at the 141st position; and depending on the C575T polymorphism within exon 6, alanine was replaced by valine at the 192nd position (Val192Ala). Therefore, it can also attract attention with its potential to lead to functional variations in the protein which will be synthesized depending on the amino acid variations in the presence of polymorphism [11].
It has been reported that allele frequencies may vary depending on the ethnic groups [16]. The C allele frequency of the C421T polymorphism was found to be 84.6 % in our control group and 82 % in our patient group. Wuyts et al. [17] reported that the RANK 421C allele frequency in healthy Caucasians was found to be 40 %. Unlike our results, in the study carried out by Choi et al. [10] C421T transition was not detected to be polymorphic in terms of the allele frequency in the Korean population. These differences in the results can be attributed to racial background and the differences in study designs.
In the study carried out by Choi et al. [10] out of 559 Korean postmenopausal women studied for RANK C575T polymorphism, the distribution of genotypes was 65 (11.6 %), 234 (41.9 %), 260 (46.5 %) for CC, CT and TT genotypes, respectively. In the Korean population, a higher rate of TT genotype and a lower rate of CC genotype were detected when compared to our study. Controversially, Kim et al. [12] found no RANK C575T polymorphism in their study in 385 Korean postmenopausal women. Inconsistency in results was attributed to ethnic background, size of the study group and statistical analysis [12]. In a study conducted by Chinese researchers, within 290 healthy male cases, the genotype distribution of the CC, CT, TT genotypes for the C575T polymorphism were 8.8, 46.1 and 45.1 %, respectively. In this population, the TT polymorphism was higher and the CC polymorphism was lower with respect to the control group in our study [13].
When it was considered in terms of the allele frequency, our findings were similar to the RANK 575C allele frequency given as 51 % in the Caucasians [17]. In the study carried out by Chinese researchers, the RANK 575C allele frequency was detected to be 33 % in healthy male controls [13].
Choi et al. [10] studied the association between OPG (163 A>G, 1181 G>C), RANK (421 C>T, 575 T>C) and ESR (1335 C>G, 2142 G>A) polymorphisms with adjusted BMDs values (for age and BMI) at the distal radius and calcaneus in 650 Korean postmenopausal women aged between 44 and 79 years. They reported that BMD at the calcaneus in individuals with the TT genotype for the C575T polymorphism was detected to be higher than the cases with the CC and CT genotypes (p: 0.017). In their study, gene–gene interactions were also analyzed, whereas no significant difference in BMD values between the RANK T575C and OPG G1181C combined genotypes was detected (p = 0.104), the BMD values at the distal radius and calcaneus in cases having TTRANK T575C/CCOPG G1181C combined genotype were detected to be higher than the cases with TTRANK T575C/GGOPG G1181C combined genotype in paired comparison (p: 0.002) [10]. These results, detected for the RANK C575T polymorphism, support the idea that genes can have an effect on BMD values for different body regions in various ethnic groups [18].
Koh et al. [11] examined the relationship between 25 different RANK gene polymorphisms and the BMD values at the femoral neck and lumbar region in 560 Korean postmenopausal women aged between 46 and 83 years. It was found that two intronic SNPs were related to low BMD values at the lumbar region [11]. Similar to our study, they reported that the C575T polymorphism was not found to have an influence on the BMD values at the femoral and lumbar regions in postmenopausal Korean women. In addition, no association was found between the RANK gene polymorphisms and fracture which was detected by using conventional radiographics and patient history. This result was explained by the fact that a clear relationship could not be established due to the reason that estimated contribution rate of heredity to fracture risk was between 25 and 35 % [19] and several environmental factors other than BMD have had important roles [20] in the risk of falling.
In a Chinese study (a patient group of 555 and a control group of 565), cases divided by their BMD values were examined in terms of RANK/RANKL/OPG gene polymorphisms and their association to BMD variations of the femur total region and the entire body [13]. Unlike our study, males and females who were in the premenopausal period were also included in this study. In control and patient groups, no significant difference was found in terms of demographic characteristics and risk factors. Significant relationships were detected between the RANK C575T polymorphism and the BMD values at the femoral total region and whole body only in males and it was reported that the cases having CTRANKC575T genotype had higher BMD values. In the same study, it was detected that the RANK C575T polymorphism was in a significant gene–gene interaction with the other examined polymorphisms of RANKL and OPG, again only in males.
The major restriction of our study is the limited number of cases for establishing the association between the BMD values and genetic polymorphism. Due to the restricted time period for the project, we could not extend the period of collecting participants. Due to the fact that we examined only women who were in the postmenopausal period, it was not possible to evaluate the intersexual difference. Besides, quantitative trait loci (QTL) can only match to a restricted extent between the female and male sex; it is known that in different sexes, different genes can be influential on BMD [21]. Studies in a larger population including both sexes are required in order to examine the effect of gene polymorphisms which may have influence on cases with different traits.
In conclusion, no significant difference was detected between groups with normal or low bone mineral density in terms of genotype, combined genotype or allele frequencies of the C421T polymorphism within exon 4 and C575T polymorphism within exon 6 in the RANK gene in 178 postmenopausal Turkish women. No significant difference between the C421T and C575T polymorphisms and the BMD values at the lumbar spine, femoral neck and femoral total regions in groups with normal and low bone mineral density within 178 postmenopausal Turkish women was detected. Further studies including both sexes and several more cases are required in order to examine the relationship between BMD values and the RANK gene polymorphisms.
References
NIH consensus statement (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795
Kanis JA, Melton LJ, Christiansen C 3rd, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141
Consensus development conference (1991) Prophylaxis and treatment of osteoporosis. Am J Med 90(1):107–110
Rosen CJ, Beamer WG, Donahue LR (2001) Defining the genetics of osteoporosis: using the mouse to understand man. Osteoporos Int 12(10):803–810
Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, Langdahl B, van Meurs JB, Mosekilde L, Scollen S, Albagha OM, Bustamante M, Carey AH, Dunning AM, Enjuanes A, van Leeuwen JP, Mavilia C, Masi L, McGuigan FE, Nogues X, Pols HA, Reid DM, Schuit SC, Sherlock RE, Uitterlinden AG (2004) Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA 292(17):2105–2114
Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Zillikens MC, Wilson SG, Mullin BH, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra BA, Pols HAP, Sigurdsson G, Thorsteinsdottir U, Soranzo N, Williams FMK, Zhou Y, Ralston SH, Thorleifsson G, van Duijn CM, Kiel DP, Stefansson K, Uitterlinden AG, Ioannidis JPA, Spector TD (2009) GEFOS (Genetic factors for osteoporosis) consortium. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151(8):528–537
Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM (2000) Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet 24(1):45–48
Yang GY, Pan TL, Xu F, Dong XH, Deng SS, Wen H (2008) Molecular genetic studies of gene identification for osteoporosis. Expert Rev Endocrinol Metab 3(2):223–267
Kahwati LC, Haigler L, Rideout S (2005) What is the best way to diagnose menopause? Clin Inq 54(11):1000–1002
Choi JY, Shin A, Park SK, Chung HW, Cho SI, Shin CS, Kim H, Lee KM, Lee KH, Kang C, Cho DY, Kang D (2005) Genetic polymorphisms of OPG, RANK, and ESR1 and bone mineral density in Korean postmenopausal women. Calcif Tissue Int 77(3):152–159
Koh JM, Park BL, Kim DJ, Kim GS, Cheong HS, Kim TH, Hong JM, Shin HI, Park EK, Kim SY, Shin HD (2007) Identification of novel RANK polymorphisms and their putative association with low BMD among postmenopausal women. Osteoporos Int 18(3):323–331
Kim JG, Kim JH, Kim JY, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM (2007) Association between osteoprotegerin (OPG), receptor activator of nuclear factor-kappaB (RANK), and RANK ligand (RANKL) gene polymorphisms and circulating OPG, soluble RANKL levels, and bone mineral density in Korean postmenopausal women. Menopause 14(5):913–918
Hsu YH, Niu T, Terwedow HA, Xu X, Feng Y, Li Z, Brain JD, Rosen CJ, Laird N, Xu X (2006) Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum Genet 118(5):568–577
Xiong DH, Shen H, Zhao LJ, Xiao P, Yang TL, Guo Y, Wang W, Guo YF, Liu YJ, Recker RR, Deng HW (2006) Robust and comprehensive analysis of 20 osteoporosis candidate genes by very high-density single-nucleotide polymorphism screen among 405 white nuclear families identified significant association and gene–gene interaction. J Bone Miner Res 21(11):1678–1695
Dong SS, Liu XG, Chen Y, Guo Y, Wang L, Zhao J, Xiong DH, Xu XH, Recker RR, Deng HW (2009) Association analyses of RANKL/RANK/OPG gene polymorphisms with femoral neck compression strength index variation in Caucasians. Calcif Tissue Int 85(2):104–112
Mori M, Yamada R, Kobayashi K, Kawaida R, Yamamoto K (2005) Ethnic differences in allele frequency of autoimmune-disease-associated SNPs. J Hum Genet 50(5):264–266
Wuyts W, Van Wesenbeeck I, Morales-Pıga A, Ralston S, Hockıng I, Vanhoenacker F, Westhovens R, Verbruggen I, Anderson D, Hughes A, Van Hul W (2001) Evaluation of the role of RANK and OPG genes in Paget ’s disease of bone. Bone 28(1):104–107
Deng HW, Li J, Li JL, Johnson M, Gong G, Recker RR (1999) Association of VDR and estrogen receptor genotypes with bone mass in postmenopausal Caucasian women: different conclusions with different analyses and the implications. Osteoporos Int 9(6):499–507
Kannus P, Palvanen M, Kaprio J, Parkkari J, Koskenvuo M (1999) Genetic factors and osteoporotic fractures in elderly people: prospective 25 year follow up of a nationwide cohort of elderly Finnish twins. BMJ 319(7221):1334–1337
Tran BN, Nguyen ND, Center JR, Eisman JA, Nguyen TV (2009) Enhancement of absolute fracture risk prognosis with genetic marker: the collagen I alpha 1 gene. Calcif Tissue Int 85(5):379–388
Ralston SH, Galwey N, MacKay I, Albagha OM, Cardon L, Compston JE, Cooper C, Duncan E, Keen R, Langdahl B, McLellan A, O’Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass J, Bennett ST (2005) Loci for regulation of bone mineral density in men and women identified by genome wide linkage scan: the FAMOS study. Hum Mol Genet 14(7):943–951
Acknowledgments
This study was supported financially by Turkish Osteoporosis Society.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
İşleten, B., Durmaz, B., Durmaz, B. et al. The association of RANK gene C421T and C575T polymorphisms with bone mineral density in postmenopausal Turkish women. Arch Gynecol Obstet 288, 917–923 (2013). https://doi.org/10.1007/s00404-013-2831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2831-y