Abstract
Purpose
It was shown that individuals homozygous for the Arg-encoding allele of codon 72 TP53 gene may have an increased risk to human papillomavirus (HPV)-related cervical carcinomas. However, many studies have failed to confirm this hypothesis. The aim of this study was to investigate a role of the TP53 codon 72 polymorphism in cervical carcinoma development in Serbian women.
Methods
In comparative, prospective study, we analyzed 49 wild type TP53 gene cervical carcinomas samples and 74 cervical smears of gynecologically healthy women. DNA was extracted by salting-out procedure. Codon 72 polymorphism was assessed by Restriction Fragment-Length Polymorphism method. Presence of HPV infection was detected through amplification of one part of L1 viral gene. χ2 and odds ratio were used for statistical analysis.
Results
The distribution of Arg/Arg, Arg/Pro, and Pro/Pro genotypes of codon 72 of TP53 gene was: 63.3, 34.7, and 2.0 % in the cervical carcinomas and 58.1, 33.8, and 8.1 % in the control group. We observed an increased risk for the development of cervical carcinoma for Arg homozygotes in relation to heterozygotes plus Pro homozygotes (OR 1.24; 95 % CI 0.59–2.61) and higher one for Arg/Arg plus Arg/Pro genotype in relation to Pro homozygotes (OR 4.24; 95 % CI 0.49–36.32).
Conclusions
The results indicate that carriers of Arg allele of codon 72 TP53 gene have an increased risk for development of cervical carcinoma in Serbian women. However, the influence is not statistically significant and requires analysis of larger case–control group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical carcinoma is the second most frequent malignancy in women worldwide. More than 85 % of cases occur in developing countries [1], mainly because of the lack of routine screening, which will enable early detection of preneoplastic cervical lesions. Serbia has one of the highest incidence rate (age-standardized) of cervical carcinoma in Europe [2].
Among cervical carcinomas, 85–90 % are of the squamous cell type and the remaining 10–15 % are of the adenocarcinomas [3]. The main factor in the development of cervical carcinomas is the infection with high-risk human papillomavirus (HPV). Integration of these viruses into the host genome results in continual expression of E6 and E7 virus oncoproteins and consequently impairs cell proliferation, DNA repair, differentiation, and apoptosis [4]. HPV DNA was detected in more than 99 % of squamous cervical carcinomas, with type 16 accounting for about half of cases [5]. In cervical adenocarcinomas, the percentage of HPV infection is lower and may be age-dependent. HPV18 is the predominant type of HPV in adenocarcinomas, followed by HPV16 [6].
The fact that only small fraction of women infected with HPV develop cervical carcinoma [7] indicates that HPV infection is not sufficient and points to the role of other cofactors. Some of them are sexual behavior, hormonal factors, smoking, outer sexually transmitted diseases, and diet [8]. Genetic susceptibility may also be important.
One of the possible genetic marker for cervical carcinoma risk is the polymorphism at codon 72 of TP53 gene. This polymorphic site encodes either an Arg amino acid (CGC) or a Pro amino acid (CCC) in proline-rich region of the P53 protein that is required for growth suppression and apoptosis mediated by P53. These two P53 variants are functionally different based on the ability to bind components of the transcriptional machinery, activate transcription, induce apoptosis, and suppress tumor growth [9]. Arg allele is more efficient in inducing apoptosis while Pro allele induces higher level of G1 arrest [10]. However, in contrast to wild type (wt) P53 Arg72, mutant P53 with Arg72 may enhance tumor growth by binding to P53-homolog P73 and neutralization of P73-induced apoptosis [11].
Potential role of the TP53 codon 72 polymorphism in cervical carcinoma risk has been suggested by the findings that P53 Arg72 variant is more susceptible to ubiquitin-dependent degradation mediated by high-risk HPV E6 protein [12]. The association of the TP53 codon 72 polymorphism and cervical carcinoma risk has been studied by different groups but with inconsistent results.
An impact of the TP53 codon 72 polymorphism in cervical carcinoma development in Serbian women has not been reported yet. The aim of this study was to investigate possible role of the codon 72 TP53 gene polymorphism for cervical carcinoma risk.
Materials and methods
Study population
This study included the cases (49 women) with histologically confirmed cervical carcinomas with previously determined wild type TP53 gene status in tumor tissue. The tumors were classified and graded according to the World Health Organization (WHO) criteria. The stages were established according to the International Federation of Obstetrics and Gynecology (FIGO) system.
The control group (74 women) was selected among women who participated in the routine gynecological examination, cytological screening, and HPV testing at the Institute for oncology and radiology of Serbia. Inclusion criteria were normal findings by gynaecological exam without any cytological abnormality and history of precancerous or cancerous lesion of pelvic region. The selection was made among 223 women who met the above criteria by the table of random numbers [13] so that: control subjects were matched to case within 10-year intervals (<40, 40–49, 50–59, >60 years) and the same ratio of HPV-infected versus HPV-uninfected individuals were obtained in 10-year age groups as in all group of women with the mentioned criteria.
All cases and controls were of Caucasian origin. The age in controls group is ranged from 31 to 74 (median 48 years), and in control group from 25 to 73 (median 49 years). Other demographical data were shown in Table 1.
The research was approved by Ethics Committee of the Institute for oncology and radiology of Serbia.
DNA extraction and detection of HPV infection
Genomic DNA was isolated by the salting-out method from cervical carcinoma tissue samples collected after surgical treatment and stored in liquid nitrogen at −197 °C (cases) and exfoliated cervical cells (control subjects).
Human papillomavirus DNA were detected by PCR amplification, using general primers GP5+ and GP6+. These primers have been designed to amplify the 150 bp conserved sequence of L1 viral gene of a broad spectrum of HPV genotypes (6, 11, 13, 16, 18, 30–35, 39, 40, 42, 45, 51–53, 56, 58, 61, 66, 68) [14]. DNA from HeLa cells containing HPV18 was used as PCR positive control. Human β globin amplification was used to test sample DNA quality. Primers’ sequences and PCR conditions were described previously [15]. Presence of amplified L1 and β globin gene were checked by electrophoresis on a 2 % agarose gel.
Genotyping of TP53 gene at codon 72
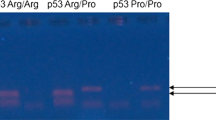
TP53 gene exon 4 was amplified by PCR. Primers’ sequences and PCR conditions were described previously [15]. Codon 72 polymorphism was assessed by the Restriction Fragment-Length Polymorphism method. 1.0 μL of each PCR product of TP53 exon 4 was digested with 1.5 μL of the restriction enzyme Bsh1236I (Fermentas, Lithuania) at 37 °C for 1 h and 20 min. The digestion reaction was stopped by heating at 65 °C for 20 min. After digestion, the fragments were separated on 8 % polyacrylamide gel for 100 min at 100 V at room temperature. Arg allele was cut by Bsh1236I in two fragments (126 and 170 bp). Pro allele was not cut by Bsh1236I and had a single 296 bp band. The heterozygote contained three bands (126, 170, and 296 bp).
Statistical analysis
Deviation from Hardy–Weinberg equilibrium in the studied groups was examined by χ2 test. p < 0.05 was considered statistically significant.
The association between TP53 codon 72 polymorphism and cervical carcinoma was determined using odds ratio (OR) and 95 % confidence interval (95 % CI).
Results
Among 49 wild-type TP53 cervical carcinomas 91.8 % were squamous cell types and 8.2 % were adenocarcinomas. The age in the case group ranged from 31 to 74, with median of 48 years. This group was in Hardy–Weinberg equilibrium in relation to codon 72 TP53 gene locus (χ 21 = 0.619, p > 0.05).
The age in the control group of 74 women ranged from 25 to 73, with median of 49 years. The control group was in Hardy–Weinberg equilibrium in relation to the investigated TP53 gene locus (χ 21 = 0.727, p > 0.05).
Human papillomavirus infection was present in 88/223 (39.5 %) women with normal gynecological and cytological findings. Range of HPV infected versus HPV non-infected women were: 46.2 versus 53.8 % in group less than 40 years, 36.0 versus 64.0 % in group of 40–49 years, 35.7 versus 64.3 % in group of 50–59 years and 41.2 versus 58.8 % in group of more than 60 years.
PCR amplification and digestion of PCR product of exon 4 of TP53 gene by Bsh1236I enzyme were successful in all patients and controls (Fig. 1).
There is a trend of increase of frequency of Arg homozygote and heterozygote in cases versus controls (Fig. 2). The distributions of codon 72 TP53 genotypes in cases versus controls were: 31/49 (63.3 %) versus 43/74 (58.1 %) for Arg/Arg, 17/49 (34.7 %) versus 25/74 (33.8 %) for Arg/Pro, and 1/49 (2.0 %) versus 6/74 (8.1 %) for Pro/Pro.
The frequencies of alleles were: 0.81 for Arg and 0.19 for Pro in cases, 0.75 for Arg, and 0.25 for Pro in controls.
There is a slightly increased risk for the development of cervical carcinoma for Arg homozygotes in relation to heterozygotes plus Pro homozygotes (OR 1.24; 95 % CI 0.59–2.61) and some higher one for carrier of Arg allele (Arg/Arg and Arg/Pro genotype) in relation to Pro homozygotes (OR 4.24; 95 % CI 0.49–36.32).
Discussion
Since 1998, when Storey et al. [12] suggested that individuals with the Arg/Arg compared to Arg/Pro codon 72 TP53 genotype have a higher risk of development of HPV-associated cancer, intensive researches were carried out worldwide regarding the polymorphic variants of TP53 codon 72 gene and occurrence of different types of cancer.
The most studied relation was the one between codon 72 polymorphism of TP53 gene and risk of cervical cancer. However, these studies were inconsistent and many of them failed to confirm the original finding of Storey and et al. Still, meta-analyses which summarized the results from different parts of the world showed slightly elevated risk for cervical cancers for Arg homozygotes compared to heterozygotes or Pro homozygotes [16–18]. In relation to histological type, there was a slightly higher risk for adenocarcinoma compared to squamous cell carcinoma [19]. Unlike cervical cancer, the association between Arg/Arg genotype and a risk of developing the squamous intraepithelial neoplasia was not shown in meta-analyses [17, 19]. This leads to the assumption that the TP53 codon 72 polymorphism has a role in progression rather than initiation of cancerous lesions.
There are several reasons for the differences among the results of the studies. The differences may originate from the small sample size and inadequate control groups [20]. Namely, meta-analyses showed that the main cause of heterogeneity among the results is deviation from Hardy–Weinberg equilibrium in the control group [19]. This discrepancy may indicate that the control group is not representative sample of the population from which it was separated, so that the distribution of genotypes does not reflect the one that is present in the population. Also, the discrepancy may be a consequence of ethnically mixed population if there are ethnic/racial differences in the frequency of genotypes in relation to the polymorphic site, as is the case with TP53 codon 72 polymorphism.
Meta-regression analyses tried to clarify the influence of other possible causes of heterogeneity of the results, such are the source of DNA, the method for genotyping and HPV status. No significant difference was shown when the source of DNA in patient group was blood compared to tumor tissue. It is consistent with the fact that loss of heterozygosity is a rare event in cervical neoplasia [19]. Also, there was no significant difference among different methods for genotyping [17, 19]. However, different types of HPV may contribute to differences in the results according to the fact that predisposition of Arg form to E6-mediated P53 protein degradation was demonstrated only for HPV types 16 and 18. It is possible that E6 oncoprotein of other high-risk HPV types has less distinction to Arg in relation to Pro P53 protein variant. Also, it was observed that the E6-mediated degradation of P53 increased slightly for HPV18 compared to HPV16 [12]. The differences in subtype and variant of HPV may affect cervical carcinoma risk [21].
Lack of determination of TP53 mutational status in tumor tissue is the one of the causes for controversial results, due to the fact that Arg72 variant may be an inhibitor or activator of tumor growth depending on whether the P53 protein is wt or mutated [22]. Polymorphisms of activators or repressors upstream of P53, as well as effectors downstream of P53 can modify susceptibility of TP53 gene polymorphism for cancer development.
In addition to the mentioned sources of different result, conclusions from meta-analyses should be accepted with some caution, because the majority of studies analyzed the European population. Some of them analyzed the populations of Asia, North and South America, and only a few African one [16–19]. Also, studies on the European population covered only the small number of countries [17]. It should be noted that the European population accounts for 11 % of the total world population, with Russian one contributing with 2 %. For this country, we have no data about association of the TP53 codon 72 gene polymorphism and development of cervical neoplasia. Furthermore, for more than two-third of European countries data do not exist. Asia contributes 60 % of the world population, with China and India accounting for 20 and 17 %, respectively.
In this study, we tried to eliminate all potential sources of result variety. The selection of the control group was as follows: the control subjects were matched with the women with cervical carcinomas within 10-year intervals (<40, 40–49, 50–59, >60 years). The ratio of HPV-infected versus HPV-uninfected control individuals within each age-interval was the same as in the entire group from which selection was made. The control group was in Hardy–Weinberg equilibrium in relation to the investigated gene locus. Unlike previous studies, we chose the samples of tumors with wt TP53, in consistence with the fact that the mutated TP53 with Arg72 variant might be an activator of tumor growth.
The results of this study showed the slightly increased risk for the development of cervical carcinoma for Arg homozygotes in relation to heterozygotes plus Pro homozygotes and some higher one for carrier of Arg allele (Arg/Arg and Arg/Pro genotype) in relation to Pro homozygotes (OR > 1). However, the statistically significant level (95 % CI) was not reached.
In addition, it is interesting that we found the frequency of HPV infection in the control group of 39.5 %. This is a rather high incidence of HPV infection compared to the average frequency of HPV infections in cytologically normal women in Europe and various parts of the world [23, 24]. However, the studies in some countries have shown similar or higher frequency of HPV infection in group of cytologically normal women [25, 26]. The probable reason for this is the current socio-economic status and inadequate health education of women in Serbia.
In conclusion, we indicate that carriers of Arg allele of codon 72 TP53 gene have an increased risk for development of cervical carcinoma in Serbian women. It should be pointed out, that this observation is not statistically significant and needs to be confirmed in larger case–control study group.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Kesić V, Jovićević-Bekić A, Vujnović M (2007) Cervical cancer screening in Serbia. Coll Antropol 31:31–36
Tjiong MY, Out TA, Ter Schegget J, Burger MP, Van Der Vange N (2001) Epidemiologic and mucosal immunologic aspects of HPV infection and HPV-related cervical neoplasia in the lower female genital tract: a review. Int J Gynecol Cancer 11:9–17
von Knebel Doeberitz M (2002) New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infection. Eur J Cancer 38:2229–2242
Burd ME (2003) Human papillomavirus and cervical Cancer. Clin Microbiol Rev 16:1–17
Andersson S, Rylander E, Larsson B, Strand A, Silfversvärd C, Wilander E (2001) The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur J Cancer 37:246–250
Nagpal JK, Sahni S, Das BR (2002) P53 codon 72 polymorphism and susceptibility to development of human papilloma virus-associated cervical cancer in Indian women. Eur J Clin Invest 32:943–948
Castellsaqué X, Bosh FX, Muñoz N (2002) Environmental co-factors in HPV carcinogenesis. Virus Res 89:191–199
Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G (1999) Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol 19:1092–1100
Pim D, Banks L (2004) p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer 108:196–199
Marin MC, Jost CA, Brooks LA et al (2000) A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat Genet 25:47–54
Storey A, Thomas M, Kalita A et al (1998) Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 393:229–234
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Inc., Englewood Cliffs
de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ (1995) The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol 76:1057–1062
Malisic E, Jakovljevic K, Jankovic R, Radulovic S (2011) TP53 gene status and HPV infection in response to platinum-plus taxane-based chemotherapy of ovarian carcinomas. J BUON 16:701–707
Jee SH, Won SY, Yun JE, Lee JE, Park JS, Ji SS (2004) Polymorphism p53 codon-72 and invasive cervical cancer: a meta-analysis. Int J Gynaecol Obstet 85:301–308
Sousa H, Santos AM, Pinto D, Medeiros R (2007) Is the p53 codon 72 polymorphism a key biomarker for cervical cancer development? A meta-analysis review within European populations. Int J Mol Med 20:731–741
Klug SJ, Ressing M, Koenig J et al (2009) TP53 codon 72 polymorphism and cervical cancer: a pooled analysis of individual data from 49 studies. Lancet Oncol 10:772–784
Koushik A, Platt RW, Franco EL (2004) p53 codon 72 polymorphism and cervical neoplasia: a meta-analysis review. Cancer Epidemiol Biomark Prev 13:11–22
Whibley C, Pharoah PD, Hollstein M (2009) p53 polymorphisms: cancer implications. Nat Rev Cancer 9:95–107
Ferenczy A, Franco E (2002) Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol 3:11–16
Pietsch EC, Humbey O, Murphy ME (2006) Polymorphisms in the p53 pathway. Oncogene 25:1602–1611
Clifford GM, Gallus S, Herrero R et al (2005) Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 366:991–998
de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX (2007) Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 7:453–459
Tábora N, Bakkers JM, Quint WG, Massuger LF, Matute JA, Melchers WJ, Ferrera A (2009) Human papillomavirus infection in Honduran women with normal cytology. Cancer Causes Control 20:1663–1670
De Vuyst H, Parisi MR, Karani A et al (2010) The prevalence of human papillomavirus infection in Mombasa, Kenya. Cancer Causes Control 21:2309–2313
Acknowledgments
The authors truly thank Gordana Kukic and Filip Stojanovic for their excellent technical assistance. This study was financially supported by the No. 41026 grant of the Ministry of Education and Science of Serbia.
Conflict of interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malisic, E., Jankovic, R., Brotto, K. et al. TP53 codon 72 polymorphism and risk of cervical carcinoma in Serbian women. Arch Gynecol Obstet 288, 621–625 (2013). https://doi.org/10.1007/s00404-013-2783-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2783-2