Abstract
Infection by high-risk human papillomavirus (HR-HPV) and single nucleotide polymorphism (SNP) in genes involved in cell cycle control, as p21 and p27, are important factors in the development of different types of human cancers. This study aims at investigating whether both the p21 Ser31Arg and p27 V109G polymorphisms are associated with susceptibility to the development of cervical lesions in women HR-HPV positive. We analyzed 132 women HPV positive and with cervical lesions or CC and 154 healthy control (HPV negative and without cervical lesions). p21 Ser31Arg and p27 V109G polymorphisms were analyzed using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method and sequencing. The p21 31Arg allele was associated with susceptibility for the development of cervical lesions (P* = 0.0009), while p27 V109G polymorphism showed no significant differences for this association (P* = 0.89). However, the combined effect of the polymorphisms showed that the presence of the CC genotype (SNP p21 Ser31Arg) conferred protection for the development of cervical lesions (OR = 0.39). p21 Ser31Arg and p27 V109G polymorphisms were not associated with the grade of cervical lesions (CINI, CINII, and CINIII) or CC (P* > 0.05). The HR-HPV more frequent in this study were of 16 (57.6 %) and 18 (37.1 %) types; however, no association was observed when both polymorphisms and risk factors analyzed were compared (P* > 0.05). Our findings suggest a possible association between p21 Ser31tabArg polymorphism and susceptibility to the development of cervical lesions in women from Pernambuco. Brazil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer (CC) represents the second most common cancer in Brazil and is the fourth leading cause of death in Brazilian women. In years 2014–2015, 15,590 new cases of CC are estimated, while in Pernambuco, 6.13 new cases per 100,000 women are expected [1]. The main etiological agent of CC is the human papillomaviruses (HPV) infection, which is found in more than 90 % of the cases [2, 3]. Among over 100 types of HPV, the 16, 18, 31, and 33 are the most frequent high-risk HPV (HR-HPVs) types found in both cervical intraepithelial neoplasia (CIN) and cervical cancer [3]. Moreover, several other factors appear to increase susceptibility to develop cervical cancer, including genetic and environmental factors (tobacco use, multiple sexual partners, alcohol consumption, and number of children) [4, 5].
The cell cycle is divided into four phases, G1, S, G2, and M, and its progression is regulated by cyclins and cyclin-depent kinases (CDK). This complex operates in the phosphorylation of proteins, such as pRb, involved in cell cycle progression [6, 7]. The pRB protein inhibits the activity of the transcription factor E2F blocking the G1 to S phase transition, when phosphorylated by the cyclin CDK complex becomes inactive, liberating the E2F factor and thus enabling the continuity of the cell cycle [8]. DNA damage in normal cells causes the activation of the cyclin-dependent kinase inhibitors (CKIs) that inhibit the cyclin-CDK complex aimed at preventing the unregulated proliferation of cells [9].
The CKIs are classified into subclasses: Kip/Cip, which include P21 and P27, and INK4 [10]. The P21, upregulated by wild-type tumor suppressor protein P53, inhibits cyclin-CDK2 or cyclin-CDK4 complexes and hinders the transition from the G1 to the S phase [7, 8]. Also, high levels of P27 inhibit cyclin E/CDK2 complex in the G1 phase and block the advance of the cell cycle [11].
The development of cancers results from the uncontrolled proliferation of cells mainly caused by interference in the cell cycle checkpoints. The HR-HPV infection increases the expression of oncoproteins E6 and E7 that inhibit the activity P21 and P27 proteins, facilitating malignant transformation [7, 12].
Single nucleotide polymorphism (SNP) of the p21 gene (C>A) at codon 31 (p21 Ser31Arg; rs1801270) produces an amino acid substitution of arginine for serine, resulting in altered levels of protein [13]. Genetic studies have found that SNPs of the p21 gene could influence on the development of several pathology, as cervical, lung, and gastric cancer [14–19]. The SNP (T>G) at codon 109 of p27 gene (p27 V109G; rs2066827), which promotes a valine-to-glycine substitution, is associated with altered activity of the protein [20]. Mutations in this tumor suppressor gene has been reported to be involved in human tumor progression, as in a colon cancer, breast cancer, oral squamous cell carcinoma, and endometriosis [21–25]. Besides, some studies suggest that decreased levels of p27 may be important in the development of cervical carcinoma, since that both a diminished expression in women with lesions and high levels of p27 in normal cervix are found [26–28]
Prior studies have related these polymorphisms with cervical cancer, but the results are conflicting and even at present moment, no study has been conducted relating these polymorphisms with the development of cervical lesion [22–25, 28]. Therefore, further studies to assess these possible associations are needed. Thus, the aim of the present work was to evaluate the possible association of the single nucleotide polymorphisms in the p21 and p27 genes with the development of cervical lesions in women with HR-HPV infection.
Methods
Study population
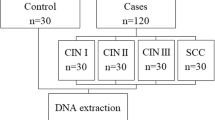
The study group consisted of 132 sexually active women from Recife metropolitan region (Pernambuco, Northeast Brazil), with ages between 16 and 75 years and mean age 33.9 ± 10.3 years, presenting HR-HPV infection and different grade of cervical intraepithelial neoplasia (low grade or CIN I, and high grade or CIN II, III) or cervical cancer, which were confirmed by cytological and histological analysis. All patients were initially assessed by colposcopy, and subsequently, cervical smears were collected. Histological diagnosis was made according to Solomon et al. [29] and Associacao Brasileira de Ginetoscopia [30]. Patients were also stratified according to smoking, alcohol consumption, multiple sexual partners, and number of children.
One hundred fifty-four unrelated women volunteers without HPV and cervical lesions from Recife metropolitan region (healthy controls group), with ages between 14 and 70 years (mean age 37.7 ± 10 years), with no history of lesions or neoplastic disease as evaluated by the physician were enrolled as controls, and written informed consent was obtained.
The study population was recruited between January 2009 and December 2009 at the Lower Genital Tract Pathology Clinic at the Women’s Healthcare Center of the Prof. Fernando Figueira Institute of Integrated Medicine, Pernambuco, Brazil. All women survey participants answered a questionnaire including social and demographic features, including age, level of instruction, age of the first sexualintercourse, number of partners, sexual behavior, smoking, and alcohol consumption. The institutional ethics and research committees (no. 355/08) approved this study.
DNA extraction
All the analyses were realized in the Laboratory of Genetic, Biochemistry and DNA Sequencing at Rural Federal University of Pernambuco. DNA was extracted from 300 μL vaginal fluid following the manufacturer’s instructions using the Wizard® Genomic DNA Purification Kit Protocol – Promega.
HPV detection and typing
HPV DNA was detected in all samples using MY09/11, GP05+, and GP06+ consensus primers following PCR protocols published elsewhere [31, 32]. Four types of HR-HPV (such as 16, 18, 31, and 33), which infect the region anogenital, were genotyped by use of specific primers and following protocols published elsewhere [33].
Analysis of the polymorphism in the p21 gene codon 31
The genotyping of the p21 Ser31Arg polymorphism was performed as described by Li et al. [34], using polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP). The following primers were used for PCR amplification: sense (5′-GTCAGAACCGGCTGGGGATG-3′) and antisense (5′-CTCCTCCCAACTCATCCCGG-3′). The amplification produced a PCR product of 272 bp that was digested by BlpI restriction enzyme at 37 °C for 4 h, generating two fragments of 183 and 89 bp when in presence of the C allele, and only one fragment of 272 bp in presence of allele A.
Analysis of the polymorphism in the p27gene codon 109
The genotyping was also performed using PCR-RFLP. The sense primer (5-TGCAGACCCGGGAGAAAG-3′) and antisense primer (5′-CTAACCCCGTCTGGC-3), as described by Li et al. [24], were used. When the product of the PCR amplification was digested with the BglI enzyme restriction for 4 h at 37 °C, three fragments of 262, 116, and 76 bp were generated when in presence of the T allele and two fragments of 377 and 76 bp when in presence of G allele.
Sequencing
To double-check PCR-RLFP genotyping analysis of the p21 Ser31Arg and p27 V109G polymorphisms, 20 % of the samples were sequenced using the MegaBACE 1000 DNA sequencer (GE Healthcare, USA). Data were collected following the parameters (Dye Set “Z”) set by the Data Collection program v1.0.1. The obtained sequence were compared with sequences available in GenBank database (www.ncbi.nlm.nih.gov) using BLAST tool and analyzed in the program BioEdit 5.0.
Statistical analysis
The associations between the p21 and p27 polymorphisms and disease were tested using the chi-squared test. Hardy-Weinberg equilibrium (HWE) test was applied to both datasets. The frequencies of alleles and genotypes were obtained by direct counting. The possible association between SNPs and cervical lesions or CC with HR-HPVs (16, 18, 31, or 33) type or risk factors, such as smoking, alcohol consumption, multiple sexual partners, and number of children, were evaluated by the odds ratio (OR) with 95 % confidence interval. Furthermore, the same test was used to analyze the combined effect of these polymorphisms. All analyses were done using the BioStat 5.0 software program.
Results
Association of the polymorphisms in the p21 and p27 genes with development of cervical lesions
The distribution of genotypes and allele frequencies of p21 Ser31Arg and p27 V109G polymorphisms among patients and healthy controls were all in accordance with Hardy–Weinberg equilibrium. For the p21 Ser31Arg polymorphism, the genotypic distribution was (76.6 % CC, 21.4 % CA, and 2.0 % AA) and (57.6 % CC, 37.1 % CA, 5.3 % AA) in cases and healthy controls, respectively. There was an increase of twofold risk for the development of cervical lesions in the patients group (OR = 2.41; P* = 0.0009) when the AA + AC genotypes were used as reference. However, no significant difference in the distribution of genotypic frequencies between the control and patient groups (27 % TT, 86 % TG, and 41 % GG and 23 % TT, 70 % TG, and 39 % GG, respectively) was observed in relation to the p27 V109G gene polymorphism (P* = 0.89; Table 1). Also, we examined the combined effect of both polymorphisms in the development of cervical lesions or CC. This assay showed that the presence of the CC genotype (SNP p21 Ser31Arg) conferred protection even when the polymorphic variants in the p27 gene (OR = 0.39; P < 0.05) are present as reported in Table 2.
Table 3 presents the correlation between of the p21 Ser31Arg and p27 V109G polymorphisms with the grade of lesion (CIN I or CIN II, III), and in their progression to CC, no significant difference was observed (P > 0.05).
Association of the polymorphisms in the p21 and p27 genes with HR-HPV infection or with risk factors for CIN and CC
The analyses of the distribution of HR-HPV types in women with cervical lesions or CC showed elevated frequency of HPV 16 and 18 types (HPV 16 = 57.6 %, HPV 18 = 37.1 %, HPV 31 = 3.0 %, HPV 33 = 1.5 %, and other HPVs = 15.2 %). Besides that, multiple infections by HPV16/18 types were present in 14.4 % of the patients. However, the genotypic frequencies of the p21 Ser31Arg and p27 V109G polymorphisms were not associated with infection by any kind of HR-HPV analyzed neither with coinfection with HPV16/18 types (Table 4). Furthermore, comparing the genotypic frequencies of both p21 Ser31Arg and p27 V109G polymorphisms with risk factors as smoking, alcohol consumption, multiple sexual partners, and number of children, no significant association was observed (Table 5).
Discussion
In the present study, the association of the single nucleotide polymorphisms in the p21 and p27 genes with development of cervical lesions in women with HR-HPV infection was examined. Functional polymorphism in the p21 gene, as p21 Ser31Arg, promoting a nonsynonymous serine to arginine substitution and so modifying the expression and/or activity of this protein [13, 35], could increase the susceptibility to different types of cancer, including cancer cervical [14–16, 36].
In the present study, it was observed that the presence of A allele in the p21 Ser31Arg polymorphism increased twofold the risk of cervical lesion. To our knowledge, until now, no other study reported this kind of association, this polymorphism, and the development of cervical lesion. However, studies as those performed by Harima et al. [14] and in Bhattacharya et al. [37] on the Japanese and Indian population, respectively, showed a risk of the A allelic variant to the development of CC. Contrarily, the work done by Ning et al. [38] reported that the A allele is more frequent in cancer-free controls, while Roh et al.[39], Tian et al. [40], and Jiang et al. [19] associated the presence of the C allele with increased risk for the development of CC. The different results for the association of the polymorphism p21 Ser31Arg with cancer cervical may be due to the genetic heterogeneity of CC in different ethnicities and/or genotypic distribution and allelic frequencies between different populations [41].
Studies that investigated expression of the P27 protein suggest that downregulation of p27 is fundamental for the development of cervical cancer, since high expression of P27 is present in quiescent cells and normal cervical squamous epithelium [42, 43]. The p27 V109G polymorphism results in the substitution of glycine for valine, which causes an alteration in the expression, activation or degradation of P27, thereby contributing to tumorigenesis [20, 24]. However, the analyses showed no significant association between the genotypes for SNP in the p27 gene (p27 V109G; rs2066827) and the development of cervical lesions. This result can be explained considering that other genes could interact and contribute to the development of the cervical lesions, which is a multifactorial trait. Following this line of reasoning, a genetic analysis combining the SNPs studied was conducted to check if a joint effect of the two polymorphisms for the development of lesions in the cervix could occur. The results showed that the presence of the CC genotype (SNP p21 Ser31Arg; rs1801270) conferred protection even when the polymorphic variants in the p27 gene are present, suggesting a possible autonomous role of the p21 gene.
The infection by HPV 16 and 18, the most common types within the Brazilian population, are a risk factor for the development of cancer [5, 44]. In this study, these types were also found as more frequent in patients with cervical lesions (57.6 and 37.1 %, respectively). Two studies conducted in Recife (capital of Pernambuco state, in Brazil) by Baldez et al. [45] and Tavares et al. [5], analyzing 213 and 142 women HPV infected, respectively, have observed that there was the prevalence of HPV 16 in more than 50 % of their samples. In contrast, a study realized at Recife by Lorenzato et al. [46] verified that among women infected by HPV, the largest prevalence was of the viral type 31 (21.4 %). Different factors could explain these conflicting results, such as the fact that these studies had been done in different periods, the characteristics of the populations analyzed (in the present study, only women with cervical lesion were analyzed) and, also, the specificity of the technique used.
No significant difference was found when a possible association between the allelic variants p21Ser31Arg and p27 V109G and susceptibility to infection by any HR-HPV was analyzed or when other risk factors to the development of cervical lesion were considered.
Conclusion
The presence of the HR-HPV infection together with the polymorphisms in p21 Ser31Arg gene are associated with the susceptibility to development of cervical lesion in women in the state of Pernambuco, Brazil.
References
BRASIL. INCA. Instituto Nacional do Câncer. Ministério da Saúde. Cancer Prevalence Estimates 2014. Available online at: http://www.inca.gov.br/estimativa/2014/sintese-de-resultadocomentarios.asp (accessed May 13, 2014).
Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152–64.
Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of Human Papillomavirus (HPV) Disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9:119.
Pei J, Jianxin L, Wen L, Xiaoxi Z, Jianxin T. Role of p53 and p21 polymorphisms in the risk of cervical cancer among Chinese women. Acta Biochim Biophys Sin. 2010;42:671–6.
Tavares MCM, Macêdo JL, Júnior SFL, Heráclio AS, Amorim MMR, Maia MMD, et al. Chlamydia trachomatis infection and human papillomavirus in women with cervical neoplasia in Pernambuco-Brazil. Mol Biol Rep. 2014;41:865–74.
Cotran RS, Kumar V, Collins T. Robbins: patologia estrutural e funcional. 6.ed. Rio de Janeiro: Guanabara Koogan. 2000
Kumar V, Abbas A, Fausto N, Mitchell RN. Robbins: patologia básica. 8.ed. Rio de Janeiro: Guanabara Koogan. (2010)
Singh S, Johnson J, Chellappan S. Small molecule regulators of Rb-E2F pathway as modulators of transcription. Biochim Biophys Acta. 2010;1799(10–12):788–94.
Eleutério Junior J, Giraldo PC, Gonçalves AK. Marcadores imunoistoquímicos de lesões precursoras do câncer de colo uterino associadas ao HPV: o papel da proteína de supressão tumoral p16ink4a. DST J Bras Doenças Sex Transm. 2006;18(1):62–5.
Knebel MD. p16INK4a as a biomarker for differentiating replicating and transforming high risk HPV infections: the theoretical concept and its potential diagnostic impact HPV today, Newsletter on Human Papillomavirus. 2009; 7–8, M– 35437–2001.
Itamochi H, Yoshida T, Walker CL, Bartholomeusz C, Aoki D, Ishihara H, et al. Novel mechanism of reduced proliferation in ovarian clear cell carcinoma cells: cytoplasmic sequestration of CDK2 by p27. Gynecol Oncol. 2011;122:641.
Bahnassy AA, Zekri AR, Saleh M, Lotayef M, Moneir M, Shawki O. The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clin Pathol. 2007;7:94–9.
Koushik A, Platt RW, Franco EL. p53 Codon 72 Polymorphism and cervical neoplasia: a meta-analysis review. Cancer Epidemiol Biomarkers Prev. 2004;13:11–22.
Harima Y, Sawada S, Nagata K, Sougawa M, Ostapenko V, Ohnishi T. Polymorphism of the WAF1 gene is related to susceptibility to cervical cancer in Japanese women. Int J Mol Med. 2001;7:261–4.
Xi YG, Ding KY, Su XL, Chen DF, You WC, Shen Y, et al. p53 polymorphism and p21WAF1/CIP1 haplotype in the intestinal gastric cancer and the precancerous lesions. Carcinogenesis. 2004;25:2201–6.
Choi YY, Kang HK, Choi JE, Jang JS, Kim EJ, Cha SI, et al. Comprehensive assessment of p21 polymorphisms and lung cancer risk. J Hum Genet. 2008;53:87–95.
Lei D, Sturgis EM, Liu Z, Zafereo ME, Wei Q, Li G. Genetic polymorphisms of p21 and risk of second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Carcinogenesis. 2010;31:222–7.
Van den Broeke C, Radu M, Chernoff J, Favoreel HW. An emerg- ing role for p21-activated kinases (paks) in viral infections. Trends Cell Biol. 2010;20:160–9.
Jiang P, Liu J, Li W, Zeng X, Tang J. Role of p53 and p21 polymorphisms in the risk of cervical cancer among Chinese women. Acta Biochim Biophys Sin. 2010;42(9):671–6.
Pasquali D, Circelli L, Faggiano A, Pancione M, Renzullo A, Elisei R, et al. CDKN1B V109G polymorphism a new prognostic factor in sporadic medullary thyroid carcinoma. Eur J Endocrinol. 2011;164(3):397–404.
Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154(2):313–23.
Huang LW, Chao SL, Hwang JL, Chou YY. Downregulation of p27 is associated with malignant transformation and aggressive phenotype of cervical neoplasms. Gynecol Oncol. 2002;85:524–8.
Kibel AS, Suarez BK, Belani J, Oh J, Webster R, Brophy-Ebbers M, et al. CDKN1A and CDKN1B polymorphisms and risk of advanced prostate carcinoma. Cancer Res. 2003;63:2033.
Li G, Sturgis EM, Wang LE, Chamberlain RM, Spitz MR, El-Naggar AK, et al. Association between the V109G polymorphism of the p27 gene and the risk and progression of oral squamous cell carcinoma. Clin Cancer Res. 2004;10:3996.
Camargo-Kosugi CM, da Silva ID, Sato H, D’Amora P, Carvalho CV, Nogueira-de-Souza NC, et al. Eur J Obstetrics Gynecology Reproductive Biol. 2009;145:180–3.
Tae KY, Kyoung CE, Hoon CN, Hung KJ, Ick Yang W, Wook KJ, et al. Expression of cyclin E and p27KIP1 in cervical carcinoma. Cancer Lett. 2000;153(1–2):41–50.
Goff BA, Sallin J, Garcia R, VanBlaricom A, Paley PJ, Muntz HG. Evaluation of p27 in preinvasive and invasive malignancies of the cervix. Gynecol Oncol. 2003;88:40–4.
Sgambato A, Zannoni GF, Faraglia B, Camerini A, Tarquini E, Spada D, et al. Decreased expression of the CDK inhibitor p27KIP1 and increased oxidative DNA damage in the multistep process of cervical carcino- genesis. Gynecol Oncol. 2004;92(3):776–83.
Solomon MR, Bamossy GJ, Askegaard S. Consumer behaviour: European perspective. London: Pearson Education Limited; 2002.
Associacão Brasileira de Genitoscopia. Nomenclatura para laudos Colposcópico. 2002; Available online at: http://www.colposcopy.org.br (accessed august 2015).
De Roda Husman AM, Walboomers JMM, van den Brule AJC, Meijer CJLM, Snijders PJF. The use of general primers GP5 and GP6 elongated at their 30 ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Vir. 1995;76:1057–62.
Manos MM, Ting Y, Wright DK, Lewis AJ, Broker TR, Wolinsky SM. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells. 1989;7:209–14.
Karlsen F, Kalantari M, Jenkins A, Pettersen E, Kristensen G, Holm R, et al. Use of multiple PCR primer sets for optimal detection of human papillomavirus. J Clin Microbiol. 1996;34:2095–100.
Li YJ, Laurent-Puig P, Salmon RJ, Thomas G, Hamelin R. Polymorphisms and probable lack of mutation in the WAF1-CIP1 gene in colorectal cancer. Oncogene. 1995;10:599–601.
Chedid M, Michieli P, Lengel C, Huppi K, Givol D. A single nucleotide substitution at codon 31 (Ser/Arg) defines a polymorphism in a highly conserved region of the p53-inducible gene WAF1/CIP1. Oncogene. 1994;9(10):3021–4.
Wang Z, Sturgis EM, Zhang F, Lei D, Liu Z, Xu L, et al. Genetic variants of p27 and p21 as predictors for risk of second primary malignancy in patients with index squamous cell carcinoma of head and neck. Mol Cancer. 2012;11:17.
Bhattacharya P, Sengupta S. Lack of evidence that proline homozygos- ity at codon 72 of p53 and rare arginine allele at codon 31 of p21, jointly mediate cervical cancer susceptibility among Indian women. Gynecol Oncol. 2005;99:176–82.
Ning W, Shizhuo W, Qiao Z, Yanming L, Heng W, Wei L, et al. Association of p21 SNPs and risk of cervical cancer among Chinese women. BMC Cancer. 2012;12:589.
Roh J, Kim M, Kim J, Park N, Song Y, Kang S, et al. Polymorphisms in codon 31 of p21 and cervical cancer susceptibility in Korean women. Cancer Lett. 2001;165:59–62.
Tian Q, Lu W, Chen H, Ye F, Xie X. The nonsynonymous single-nucleotide polymorphisms in codon 31 of p21 gene and the susceptibility to cervical cancer in Chinese women. Int J Gynecol Cancer. 2009;19:1011–4.
Birgander R, Sjalander A, Saha N, Spitsyn V, Beckman L, Beckman G. The codon 31 polymorphism of the p53-inducible gene p21 shows dis- tinct differences between major ethnic groups. Hum Hered. 1996;46:148–54.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Stah M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. JImmuno. 2002;168:5024–31.
Oliveira-Silva M, Lordello CX, Zardo LM, Bonvicino CR, Moreira MA. Human papillomavirus in Brazilian women with and without cervical lesions. 2011; Virol J 8:4.
da Silva MF B, Chagas BS, Guimarães V, Katz LM, Felix PM, Miranda PM, et al. HPV31 and HPV33 incidence in cervical samples from women in Recife, Brazil. Genet Mol Res. 2009;8:1437–43.
Lorenzato F, Ho L, Terry G, Singer A, Santos LC, De Lucena Batista R, et al. The use of human papillomavirus typing in detection of cervical neoplasia in Recife (Brazil). Int J Gynecol Cancer. 2000;10:143–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
The work was performed at the Rural Federal University of Pernambuco (UFRPE), Recife, PE, Brazil.
Rights and permissions
About this article
Cite this article
Lima, G., Santos, E., Angelo, H. et al. Association between p21 Ser31Arg polymorphism and the development of cervical lesion in women infected with high risk HPV. Tumor Biol. 37, 10935–10941 (2016). https://doi.org/10.1007/s13277-016-4979-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4979-0