Abstract
Aim
To evaluate whether the presence of specific polymorphism in the gene promoter of collagen and some matrix metalloproteinases was associated with the risk of developing pelvic organ prolapse.
Methods
A case–control study was carried on 233 women: 137 were cases with ≥stage II pelvic organ prolapse and 96 were matched controls without pelvic pathologies. Allele and genotype frequencies related to polymorphisms at the Sp1 site of type I collagen and some functional polymorphisms in the promoters of metalloproteinases-1, -3 and -9 have been compared between groups. It has been shown that these single-insertions/deletions polymorphisms located in the promoter region of the genes have a functional significance in the regulation of their transcriptional level and local expression. Genotypes were determined by polymerase chain reaction (PCR) amplification and sequence analysis. SPSS 14.0 software was used for data analysis. Probability values of <0.05 were considered statistically significant.
Results
No difference between groups was found in the genotype distribution polymorphisms for COL1A1, metalloproteinases-9 and -3, while the distribution of the polymorphism of metalloproteinases-1 was significantly increased in the cases when compared with controls (p = 0.04).
Conclusions
Our findings suggest that the polymorphism of metalloproteinases-1 might have a role in mediating susceptibility to pelvic organ prolapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) and urinary incontinence are major health problems in women in the reproductive and menopausal years, being associated with an 11% lifetime risk of having an operation for the disease [1]. In the USA, more than 200,000 women every year are operated on for such a disease and up to 30% of patients require a new operation to solve their problem, with increasing health services and social costs [2]. The etiology of POP is multifactorial and several risk factors such as advancing age, childbearing, obesity, hysterectomy, instrumental delivery and menopause have been identified [3, 4]. Recent evidence suggests that POP has a certain genetic component with a 3.2 and 2.4 increased risk in mothers and sisters of affected women, respectively [5]. The importance of the genetic background is also suggested by the high prevalence of the disease in patients affected by disorders of type I and III collagen such as Ehlers–Danlos and Marfan syndromes [6].
Collagen amount and type and degree of crosslinking contribute to the properties and strength of the connective tissue. Continuous tissue remodeling makes the relationship between the synthesis of collagen and its degradation critical to the maintenance of tensile strength. Collagen is a fibrous protein presents in 19 types with types I and III being the main structural components of the connective tissue. Collagen fibers are stabilized by intermolecular covalent crosslinks by pyridinoline, and their degradation depends on the activity of proteinases secreted by connective-tissue cells, including the matrix metalloproteinases (MMPs) and the cathepsins. It has therefore been suggested that increased degradation of collagen may lead to a decrease in the mechanical strength, thus predisposing to POP. In particular, pelvic organ prolapse was shown to be associated with a 25% loss of collagen content in tissues. Different types of MMPs are present at different anatomic sites. MMP-1 is an interstitial collagenase, whose primary substratum is fibrillar collagen type I, II, III and IV and it is mainly localized in genital tissue, colon and vessels. MMP-9 and MMP-3 are stromelysins (gelatinase-B and stromelysin-1) whose substrata are proteoglycans, laminin and fibronectin that are mainly present in vessels and in pelvic tissue. It has been recently demonstrated by immunohistochemistry that in uterosacral ligaments and in vaginal tissue of women with POP there is an increased expression of MMP-1, MMP-2 and MMP-9 [7–9].
The genetic expression of these proteins depends on several factors, in particular on different polymorphisms in the promoter site. Even though the majority of these polymorphisms are usually “inactive”, a part of them might be associated with some differences in transcription and/or expression of proteins, which are then linked to some individual differences in diseases development [10]. To contribute to the analysis of the genetic background of POP, we evaluated the frequency of functional polymorphisms in candidate MMP and collagen genes in the serum of a population of Caucasian women affected by this condition. Specifically we have studied:
-
1.
The point mutation (G–T) responsible for the Sp1 site polymorphism in the first intron of COL1A1 gene; this mutation has been shown in subjects with Ehlers–Danlos disease [6].
-
2.
A single-insertion polymorphism (2G) in the MMP-1 promoter region known to elevate transcriptional level and local expression of MMP-1. A major expression of MMP-1 due to this polymorphism has been shown in some oncological diseases (ovarian, endometrial and colon-rectal cancer) [11].
-
3.
A single-insertion/deletion polymorphism (5A/6A) located in the promoter of the MMP-3 gene; the 5A allele is associated with a major transcription of MMP-3 in fibroblasts and in smooth muscle cells [12].
-
4.
The MMP-9 polymorphism is due to a C instead of a T and larger presence of C determines a major transcription and therefore a major expression of MMP-9. A major expression of MMP-9 has been shown in women with low bone density [13].
Genotypes were determined by polymerase chain reaction (PCR) amplification and sequence analysis.
The frequency of these variants in women with POP has been compared with that of women from the same geographic area in whom a diagnosis of disorder was clinically ruled-out.
Materials and methods
From January 2004 to June 2006, 233 women who attended the urogynecologic unit and the outpatient menopausal clinic of the departments were enrolled in the study. All women underwent a structured interview and physical examination. Pelvic floor defects were determined using the pelvic organ prolapse quantification system (POP-Q system) [14]. Measurements were made at different vaginal sites (anterior and posterior vagina and cervix) with the patient recumbent and straining down.
With the POP-Q system, each vaginal site can be defined as normal (stage 0), protruding within 1 cm above the level of the hymen (stage I), between ≤1 cm proximal or distal to the plane of the hymen (stage II), protruding more than 1 cm below the plane of the hymen but less than the total vaginal length −2 cm less (stage III), or complete eversion of the total length of the affected site (stage IV).
Cases (137) considered were women with ≥stage II pelvic organ prolapse while controls (96) were those defined as having stage 0–1. Controls were matched for POP risk factors such as age, BMI, smoking habits, parity and rate of instrumental deliveries. Previous hysterectomy and/or surgery for POP or urinary incontinence as well as a past history of malignancy were exclusion criteria. Genotype frequencies related to polymorphisms at the Sp1 site of the COL1A1 gene, −1562C/T of the MMP-9 gene, −1607 1G/2G of the MMP-1 gene and −1171 5A/6A of the MMP-3 gene have been compared between cases and controls.
The patients were informed about the study protocol, procedures, and approval was granted by the local Ethic Committees.
Molecular analysis of the Sp1 site polymorphism of COL1A1 gene
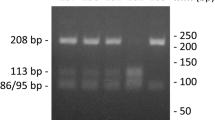
Genomic DNA was isolated from 1 ml whole blood collected in edetic acid. The point mutation (G–T) responsible for the Sp1 site polymorphism in the first intron of COL1A1 gene was detected by PCR from genomic DNA, using the following primers: P1, 5′-GGAA-GACCCGGGTTATTGCT-3′ (forward); P2, 5′-CGCTGAAGCCAAGTGAAATA-3′ (reverse) [15]. PCR products were then subjected to digestion with the restriction enzyme Van91I (New England BioLabs, Beverly, MA, USA). PCR was performed in a total volume of 25 μl containing genomic DNA, 5 pmol of each primers, 1× Taq polymerase buffer and 0.5 U of Red Taq (Sigma). Cycling conditions were set as follows: one cycle at 94°C for 5 min, 35 cycles at 94°C for 30 s, 57°C for 30 s, and 72°C for 30 s, and one final cycle of extension at 72°C for 5 min. The restriction enzyme Van91I cuts the 598 base pair (bp) PCR product in two bands of 430 and 168 bp, which were resolved on a 2.5% agarose gel.
The presence of the restriction site on both alleles was defined as homozygote G/G and the absence of the restriction site as homozygote T/T and heterozygote G/T, respectively. Cases with T/T or G/T genotypes were always confirmed at least twice. Moreover, for at least five of them, the nucleotide sequence of the PCR products was confirmed by sequence analysis.
Molecular analysis of the MMP9 gene polymorphism −1562C/T, MMP1 gene polymorphism −1607 1G/2G and MMP3 gene polymorphism −1171 5A/6A
The genotypes related to −1562C/T of MMP9, −16071G/2G of MMP1 and of −1171 5A/6A of MMP3 were determined by PCR from genomic DNA, using the following primers: 5′-GCCTGGCACATAGTAGGCCC-3′; 5′-CTTCCTAGCCAGCCGGCATC-3′ for MMP9, 5′-CCCTCTTGAACTCACATGTTATG-3′ and 5′-ACTTTCCTCCCCTTATGGATTCC-3′ for MMP-1 and 5′-TCCTCATATCAATGTGGCCAAA-3′ and 5′-CGGCACCTGGCCTAAAGAC-3′ for MMP3 [16]. PCR products were then subjected to digestion with the restriction enzyme BbuI (New England BioLabs, Beverly, MA, USA).
PCR was performed in a total volume of 25 μl containing genomic DNA, 5 pmol of each primers, 1× Taq polymerase buffer and 0.5 U of Red Taq (Sigma). Cycling conditions were set as follows: one cycle at 94°C for 5 min, 35 cycles at 94°C for 30 s, 57°C for 30 s, and 72°C for 30 s, and one final cycle of extension at 72°C for 5 min. The amplification was verified on an agarose gel (2%) followed directly by sequencing with an automatic sequencer in fluorescent DNA capillary electrophoresis (ABI PRISM 310 Genetic Analyzer; Applied Biosystems, Foster City, CA, USA).
The MMP-9 polymorphism is due to a C instead of a T, so the presence of the restriction site on both alleles was defined as homozygote C/C and the absence of the restriction site as homozygote T/T and heterozygote C/T, respectively. More presence of C induces a major transcription, so a major expression of MMP-9.
The MMP-1 polymorphism is due to an insertion/deletion of a guanine so that two allele are detected, one having a single guanine (1G) and the other having two guanines (2G). Thus, all subjects were divided into three genotypes: 1G/1G, 1G/2G and 2G/2G. The MMP-3 polymorphism is due to the variation in the length of a polymonomeric track of adenines resulting in one allele having five adenines (5A) and the other having six adenines (6A). Subjects were divided into three genotypes: 5A/5A, 5A/6A and 6A/6A. More “G” for MMP-1 and more “A” for MMP-3 induce a major transcription, so a major expression of these proteases.
Statistical analysis
Continuous variables were presented as means and standard deviation. Categorical variables were presented as numbers and percentages. Significances were tested using the t-test, the Chi-square or the Fisher exact test when appropriate. The sample size was calculated on the basis of the reported 15–20% rate of prevalence of the mutated alleles in the general population [17, 18]. Considering 80% of statistical power to avoid a type II error and 5% level of significance, we sought to find at least a twofold increase in risk of developing pelvic organ prolapse in women with genetic polymorphism. Statistical analysis was done using the SPSS statistical package (SPSS 14.0, Chicago, IL). Probability values of <0.05 were considered statistically significant.
Results
Population’s characteristics are summarized in Table 1. Family history showed that 35% of patients with POP had a first-degree relative affected by the same disease compared with a prevalence of 16% in the control group (p = 0.002; OR: 2.9, 95% CI 1.5–5.7) (Table 2).
The distribution of prolapse in affected patients at each vaginal compartment is shown in Table 3.
Women were examined before the results of genetic analysis were known, therefore, clinicians were masked to the genotypes of the individuals. The frequencies of the rarer alleles of the COL1A1, MMP-1, MMP-3 and MMP-9 polymorphisms in our control population were similar to those reported by Biondi et al. [17] and by Szyllo et al. [18].
Table 4 shows genotype for COL1A1 and MMP-9 polymorphisms in patients and controls. In both groups of women, the genotype frequency pattern showed the dominance of the unchanged homozygote for both polymorphisms (G/G for COL1A1 and C/C for MMP-9 polymorphisms). No difference was found in the genotype distribution polymorphisms between cases and controls for both COL1A1 (p = 0.16) and MMP-9 (p = 0.64). We also analyzed genotype distribution for MMP-1 and MMP-3 polymorphism (Table 4). Again there was no difference in the genotype distribution polymorphisms for MMP-3 (p = 0.53), while a greater distribution and expression of the polymorphism of MMP-1 were found in cases when compared with controls (p = 0.04).
Discussion
Pelvic organ prolapse is a debilitating disorder that affects quality of life of ageing women. The cause of this condition is far to be completely elucidated even if several risk factors have been studied [19, 20]. In recent years, there was growing evidence showing that POP is otherwise a genetic disease. The importance of the genetic background has been suggested by the high prevalence of the disease in patients affected by disorders of type I and III collagen such as the Ehlers–Danlos, and the Marfan syndromes [6]. Recently, it has been reported that POP segregated in dominant fashion with incomplete penetrance in some families. Both maternal and paternal transmissions have been observed. The relative risk in siblings of affected patients to develop POP has been shown to be five times higher when compared with that for the general population [21]. According to the previous studies we found that women who had had the mother or a sister with POP had an increased risk to develop the same disease.
Current information supports the hypothesis that extracellular matrix protein turnover plays a role in the pathogenesis of POP; most studies examining collagen and elastin expression showed a trend towards decreased contents in pelvic floor of women with pelvic floor disease being MMPs and their genetic up-regulation probably responsible for the predisposition of certain groups of women [22].
Few studies focused on the different quantity and type of collagen fibers in pelvic tissue. Gabriel et al. [23] found that major expression of type III collagen in specimens from the uterosacral ligaments was significantly associated with POP, and that it might be a typical characteristic of the connective tissue of patients with such a disease. Regarding collagen content, no difference was seen between patients with or without POP and the same finding has been shown for pre- and postmenopausal affected women. Ewies et al. [24] reported an increase in type III collagen in the cardinal ligaments of patients with pelvic organ prolapse, and also for type I collagen a modification of the quality of fibers was demonstrated. Patients with POP had shorter and thinner fibers with large spaces between, whereas in controls, fibers were longer and thicker [25].
Rather than investigating the characteristics of different types of collagen directly in tissues, we focused our attention on the hypothesis that the presence of polymorphism in the promoter gene could change the transcription of collagen and lead to an increase risk of pelvic prolapse. In our study no difference in the genotype distribution polymorphisms for COL1A1 has been found when comparing cases and controls, and this finding was in agreement with that of a recent study by Cho et al. [26] in Korean women.
The role of metalloproteinases in the pathogenesis of POP has been poorly investigated. MMPs are the key regulators of connective tissue degradation and therefore are involved in myriad of physiologic and pathologic processes, including tissue remodeling, morphogenesis, wound healing, tumor invasion, and tumor metastasis [27, 28]. In addition, they regulate many biologic processes through the release, activation or sequestration of growth factors, growth factors binding proteins, cell surface receptors and cell–cell adhesion molecules. Different types of MMPs are present at several anatomic sites. MMP-1 is an interstitial collagenases and it’s more localized in genital tissue, colon and vessels. MMP-9 and MMP-3 are stromelysins (gelatinase-B and stromelysin-1) whose substrata are proteoglycans, laminin and fibronectin that are mainly present in vessels. The fibrillar collagens are initially degraded into three-quarter and one-quarter fragments by MMPs-1, -8, and -13 before their further breakdown into soluble fibers by MMPs-2 and -9. In a previous study of vaginal wall biopsies, the expression of MMP-1 mRNA was found to be increased in patients with POP and incontinence, whereas the expression of TIMP-1 mRNA was decreased [29]. Gabriel et al. [7] found an increased MMP2 expression in the uterosacral ligaments of patients with POP, and Jackson et al. [30] examining the vaginal epithelium of eight premenopausal women with prolapse (with and without urinary incontinence) and the same tissue of ten subjects without the disease found a decrease in total collagen content and an increase in MMPs expression in the former group. Recently, it has been also demonstrated an increased expression of MMP-1 and -9 in the uterosacral ligaments and vaginal tissue of women with POP [8, 9].
Women with pelvic floor disease exhibit an important remodeling of extracellular matrix by different proteins and their inhibitors, therefore we sought to study the polymorphic variants of genes encoding these proteins, which may be considered as promoters of appearance and/or progression of the disease.
Several studies have been done looking at the existence of different polymorphic sites in promoter genes of different MMPs in women with POP with contradictory results [31, 32].
In the present study, a significant difference in the genotype frequencies of MMP-1 between cases and controls has been found, but the same was not for COL1A1, MMP-3 and MMP-9. The increase expression of MMP-1 in patients with prolapse could represent the sign of tissue remodeling to accommodate a progressively increasing mechanical load.
This is the first study that evaluated together the possibility that gene variants of collagen type I and MMPs might be involved in the susceptibility to pelvic organ prolapse.
Potential limitations included: the low number of our controls and the possible existence of other polymorphisms of these genes or other MMPs genes that might affect susceptibility to POP together with the possibility that the expression of some tissue inhibitors of metalloproteinase (TIMPs) might have a major role in the predisposition to pelvic organ prolapse.
References
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E (2002) Pelvic organ prolapse surgery in the United States. Am J Obstet Gynecol 186:712–716
Kim CM, Jeon MJ, Chung DJ, Kim SK, Kim JW, Ba SW (2007) Risk factors for pelvic organ prolapse. Int J Gynecol Obstet 98:248–251
Nygaard I, Bradley C, Brandt D (2004) Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol 104:489–497
Chiaffarino F, Chatenoud L, Dindelli M, Meschia M, Buonaguidi A, Amicarelli F et al (1999) Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol and Reprod Biol 82:63–67
Carley ME, Schaffer J (2000) Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers–Danlos syndrome. Am J Obstet Gynecol 182:1021–1023
Gabriel B, Watermann D, Hancke K, Gitsch G, Werner M, Tempfer C et al (2006) Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol 17:478–482
Vulic M, Strinic T, Bukovic D, Tomic S, Zupic T, Pavic M et al (2010) Expression of matrix metalloproiteinase-1 in uterosacral ligaments tissue of women with genital prolapse. Coll Antropol 34:1411–1414
Dviri M, Leron E, Dreiher J, Mazor M, Shaco-Levy R (2011) Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 156:113–117
Strittmatter WJ, Roses AD (1995) Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci 92:4725–4727
Dunleavey L, Beyzade S, Ye S (2000) Rapid genetype analysis of the matrix metalloproteinases 1 gene 1G/2G polymorphism that is associated with risk of cancer. Matrix Biol 19:175–177
Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM (1995) Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary aterosclerosis. Br Heart J 73:209–215
Yamada Y, Ando F, Niino N, Shimokata H (2004) Association af a polymorphism of the matrix-metaloproteinase-9 gene with bone mineral density in Japanese women. Metab Clin Ex 53:135–137
Bump RC, Mattiasson A, Bo K, Brubaker L, DeLancey JOL, Klarskov P et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Vinkanharju A, Melkko T, Risteli J, Risteli L (2001) New PCR-based method for the Sp1 site polymorphism in the COL1A1 gene. Clin Chem Lab Med 39:624–636
Minematsu N, Nakamura H, Tateno H, Nakajima T, Yamaguchi K (2001) Genetic polymorphism in matrix metalloproteinase-9 and pulmonary emphysema. Biochem Biophys Res Commun 289:116–119
Biondi ML, Ghilardi G, Mangoni J, Scorza R, Leviti S, Guagnellini E (2001) Matrix metalloproteinase-1 promoter polymorphism 1G/2G is correlated with colorectal cancer invasiveness. Clin Canc Res 7:2344–2346
Szyllo K, Smolarz H, Makowska H, Niewiadornski M, Kozlowska E, Kulig A (2002) The promoter polymorphism of the matrix metalloproteinase 3 [MMP-3] gene in women with ovarian cancer. Exp Clin Cancer Res 21:357–362
Luber KM, Boero S, Choe JY (2001) The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol 184:1496–1503
Tegerstedt G, Miedel A, Maehle-Schmidt M, Nyrén O, Hammarström M (2006) Obstetric risk factors for symptomatic prolapse: a population-based approach. Am J Obstet Gynecol 194:75–81
Jack GS, Nikolova G, Vilain E, Raz S, Rodríguez LV (2006) Familiar transmission of genitovaginal prolapses. Int Urogynecol J 17:498–501
Campeau L, Gorbachinsky I, Badlani GH, Andersson KE (2011) Pelvic floor disorders: linking genetic risk factors to biochemical changes. BJU Int 108:1240–1247
Gabriel B, Denschlag D, Göbel H, Fittkow C, Werner M, Gitsch G et al (2005) Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J 16:475–479
Ewies AA, Al-Azzawi F, Thompson J (2003) Changes in extracellular matrix proteins in the cardinal ligaments of postmenopausal women with or without prolapse: a computerized immunohistomorphometric analysis. Hum Reprod 18:2189–2195
Barbiero EC, Sartori MG, Girão MJ, Baracat EC, de Lima GR (2003) Analysis of type I collagen in the parametrium of women with and without uterine prolapse, according to hormonal status. Int Urogynecol J 14:331–334
Cho H, Jung H, Kim S, Choi J, Cho N, Bai S (2009) Polymorphism of COLIA1 gene Sp1 binding site in Korean women with pelvic organ prolapse. Yonsei Med J 50:564–568
Woessner JF Jr (1994) The family of matrix metalloproteinases. Ann N Y Acad Sci 732:11–21
Hojilla CV, Mohammed FF, Khokha R (2003) Matrix metallproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer 89:1817–1821
Chen BH, Wen Y, Li H, Polan ML (2002) Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J 13:80–88
Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ (1996) Changes in the metabolism of collagen in genitourinary prolapse. Lancet 347:1658–1661
Chen HY, Lin WY, Chen YH, Chen WC, Tsai FJ, Tsai CH (2010) Matrix metalloproteinase-9 polymorphism and risk of pelvic organ prolapse in Taiwanese women. Eur J Obstet Gynecol Reprod Biol 149:222–224
Skorupski P, Miotła P, Jankiewicz K, Rechberger T (2010) MMP-1 and MMP-3 gene encoding polymorphism and the risk of the development of pelvic organ prolapse and stress urinary incontinence. Ginekol Pol 81:594–599
Conflict of interest
The authors have no financial, personal, political, intellectual or religious interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrari, M.M., Rossi, G., Biondi, M.L. et al. Type I collagen and matrix metalloproteinase 1, 3 and 9 gene polymorphisms in the predisposition to pelvic organ prolapse. Arch Gynecol Obstet 285, 1581–1586 (2012). https://doi.org/10.1007/s00404-011-2199-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-011-2199-9