Abstract
Cervical cancer is one of the most common and lethal gynecological malignancies in both developing and developed countries, and therefore, there is a considerable interest in early diagnosis and treatment of precancerous lesions. Although the current standard care mainly based on cytology and colposcopy has reduced rates of cervical cancer morbidity and mortality, many lesions are still missed or overcalled and referred for unnecessary biopsies. Optical imaging technologies, spectroscopy approaches and high-resolution imaging methods are anticipated to improve the conventional cervical cancer screening providing in vivo diagnosis with high sensitivity and specificity. Their concept is that morphologic and biochemical properties of the cervical tissue are altered in response to its malignant transformation. In addition, contrast agents that target against specific neoplastic biomarkers can enhance the effectiveness of this new technology. Due to the unprecedented growth of these optical techniques accompanied probably by favorable cost-effectiveness, the primary detection of premalignant lesions may become more accessible in both the developing and the developed countries and can offer see-to-treat workflows and early therapeutic interventions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the second most common cancer among women and the third most common cause of cancer-related deaths; more than 80% of such cases occur in developing countries probably due to the inadequate preventative policy [1]. Invasive cervical cancer is preceded by a state of dysplasia, or cervical intraepithelial neoplasia (CIN) or squamous intraepithelial lesion (SIL) which occur in much younger women [1]. Despite the recent introduction of FDA [Food and Drug Administration]—approved prophylactic vaccines against high risk HPV types, several factors as the significant cost of vaccines, its uncertain longevity, the fact that they do not cover all oncogenic HPV subtypes, and that older women are not vaccinated, justify the necessity of routine cervical cancer screening for the foreseeable future even for vaccinated women [2].
Currently, the Papanicolaou (Pap) examination of exfoliative cells remains the primary screening tool; while its specificity is estimated to be 95–98%, the sensitivity is lower than 50% rather than 60–85% as reported previously [3]. Abnormal cervical cytology is usually followed by colposcopy which is efficient in detecting the location but often inaccurate in diagnosing the severity of the lesion. A meta-analysis of the diagnostic value of colposcopy reported high sensitivity (87–99%) but a relatively low specificity (23–87%) [4]. Biopsy and histological evaluation of the colposcopically suspicious (visually abnormal) areas are the next steps in the diagnostic approach [5], however, this is a subjective, time consuming, costly and labor intensive procedure. Visual approaches including cervicography, speculoscopy, polarprobe, and visual inspection with acetic acid (VIA) have been introduced to enhance precancer detection [5, 6].
Recent advances in fiber optics, sources, detectors and computer-controlled instrumentation have motivated the research interest regarding the clinical applications of biophotonics in the early detection of precancer in several organs including the uterine cervix. The concept is that a light with specific properties interacts with a tissue with its unique characteristics so the light–tissue interaction conveys valuable information. Optical techniques provide a real-time objective diagnosis and detect small lesions obviating biopsies. The FDA specifies four uses for optical technologies in the diagnosis and screening of cervical neoplasia: (1) to localize biopsies as an adjunct to colposcopy, (2) to triage patients after an atypical Pap smear, (3) as an adjunct to cervical or vaginal cytology, and (4) as a primary screening in the place of cervical or vaginal cytology [7]. This review presents the current status and the future perspectives of optical assessment of cervical neoplasia with the aid of spectroscopy and direct high-resolution imaging.
Optical properties of the cervix
Cancer-related changes in tissue architecture, cell morphology, vascularization and metabolic activity cause a broad range of alterations in tissue optical properties (absorption, scattering and fluorescence properties) that could function as probing targets [8]. When light strikes a tissue, it can be absorbed with or without remission or scattered by surface or subsurface interactions. An experimental study demonstrated that up to 40% of cellular scattering arises from nuclei and backscattering at angles greater than 1100 correlates with the DNA content of the cells [9]. Vessels at different depths of the cervical surface become visible with the use of different wavelengths, particularly with green light [8]. The application of acetic acid elevates the mean scattering coefficient of precancerous tissue approximately three times that of normal epithelium making abnormal tissue appear whiter than normal as a result of altered nuclear morphology, optical density, and changes in chromatin texture [10, 11] (Fig. 1). Also, cervical premalignancies are characterized by decreased stromal scattering owed to degradation in collagen fibers by neoplastic cells secreted proteases [8].
Cervical images of a patient with CIN II, capture before (a and c) and after (b and d) acetic acid application. Elimination of regular reflection (c and d) enables the visualization of abnormal (whitened) areas (d) [33]
Additionally, cervical epithelial cells present cytoplasmic autofluorescence on account of electronic excitation of mitochondrial NADH and FAD observed strongly in basal epithelial cells, and cytoceratin-induced peripheral autofluorescence upon exposure to certain wavelengths of UV and visible light [12]. In normal epithelium, basal epithelial cells show strong cytoplasmic fluorescence, while parabasal, intermediate and superficial cells show only peripheral fluorescence. During neoplastic process, cytoplasmic fluorescence becomes more dominant than peripheral fluorescence and spreads through the total thickness of epithelium according to the dominant theory of dysplastic development in the cervix where abnormal cells originating from the basal region invade the rest of the epithelium [12]. Stromal fluorescence originating from collagen and elastin crosslinks is significantly diminished possibly due to decomposition of the collagen fibers even from the very earliest stages of carcinogenesis; this reaction is influenced by covariates such as age and menopause [12]. Overall, the way that light is reflected and scattered is affected by cell and nuclear morphology and different amounts of fluorescent constituents would show semi-quantitative emission patterns.
In vivo optical spectroscopy
In vivo optical spectroscopy enables a real-time visualization of cervical tissue by fiber optic probes gleaning accurate information about structural and metabolic changes related to carcinogenesis [13]. There are two different approaches: (i) the point probe interrogation for specific cervical sites measuring 2–3 mm in extent and (ii) multispectral imaging for the entire cervix [13]. Although, the majority of clinical studies focus on fluorescence and reflectance spectroscopy, this review presents additional variables in data acquisition techniques (Tables 1, 2).
Point-probe optical spectroscopy
Reflectance spectroscopy evaluates the intensity of back scattered light correlated to illumination wavelength and provides information about alterations regarding mean nuclear diameter, nuclear size distribution, refractive index related to chromatin content and angiogenesis [13]. This optical approach with the aid of discrimination algorithms presents high sensitivity and specificity suggesting that it might be a clinical effective adjunct to colposcopy [14]. The combination of point-probe reflectance with fluorescence spectroscopy or the development of novel fiber optic probes and analysis strategies of spectral data are anticipated to offer even better classification accuracy [15, 16]. Another alternative is steady-state diffuse reflectance spectroscopy in which light delivered to the tissue surface undergoes multiple elastic scattering and absorption, and part of it returns as diffuse reflectance carrying quantitative information about subsurface substances in addition to surface reflection [17]. In a small trial examining the combination of diffuse reflectance spectroscopy with fluorescence spectroscopy, the two techniques seem to provide complementary diagnostic information [17].
Fluorescence spectroscopy is a viable optical technique that detects metabolic alterations and epithelial-stromal interactions with the potential aid of photosensitizing agents and subsequently evaluates them based on analytic models of fluorescence spectral data [18]. Precancerous lesions present lower fluorescence intensity and their peak emission wavelength is shifted to longer emission wavelengths relative to that of normal one, as a result of increased hemoglobin absorption and mitochondrial fluorescence [19, 20]. Defects of this mode include the false positive fluorescence measurements derived from inflammatory lesions that mimic a precancer-like loss in stromal fluorescence, and the insufficiency of current optical algorithms to discriminate precancers at the squamo-columnar junction as the fluorescence of columnar normal tissue and metaplasia are lower than that of squamous normal tissue [20]. A review by Mitchell et al. (1999) highlighted the predominate diagnostic ability of fluorescence spectroscopy over conventional techniques as colposcopy and subsequent studies confirmed these results [19, 21, 22]. A combined fluorescence and reflectance spectroscopy study demonstrated that fluorescence provided the best discrimination among normal and precancerous tissue compared to reflectance modality as well as to the combination of both except in the case of discriminating HG-SILs from columnar normal tissue where reflectance mode is optimal [15, 23]. Moreover, Georgakoudi et al. [16] showed that intrinsic fluorescence combined with the evaluation of reflectance spectra from the same tissue area can significantly improve precancer detection.
In order to overcome some limitations of fluorescence modality, vibrational spectroscopy including Raman and Infrared spectroscopy may be beneficial [24]. Mid-infrared spectroscopy (IR) evaluates the wavelength-dependent absorption properties by probing vibrating energy levels of functional groups and molecular interactions with an IR active dipole. Fourier transform infrared (FTIR) spectroscopy is the standard IR method today. Neoplastic alterations include glycogen reduction, increased hydrogen bonding from phosphodiester groups, loss of hydrogen bonding in alcoholic groups of amino acids and altered peak ratios of glycogen/phosphate and RNA/DNA [24]. The interpretation of the shape of a spectrum or peak ratios depends on the experience of the spectroscopist who should also be familiar with the spectral signatures of immature, benign cell types that can obscure abnormal cells. Except from precancer detection, IR spectroscopy could also be implicated in monitoring cell proliferation or/and drug response [25]. Novel approaches as the combination of FTIR microspectroscopy with multivariate spectral processing methods that recognize fundamental optical signatures of individual cells may improve further the differentiation accuracy of the method [26].
Finally, Raman spectroscopy (RS) implies the measurement of inelastically scattered light due to the energies of molecular vibrations characterizing molecules according to their change in polarity [27]. Raman signals from tissue are weak and occur, for applied wavelengths and powers, statistically only one time for 1010 incident photons. A recent study demonstrated that in vivo Raman spectra in the high wave-number region (2,800–3,700 cm−1) yielded a high diagnostic effectiveness for dysplasia identification [28]. Although near IR light would penetrate deeper into tissue, a good Raman signal-to-noise ratio from cervical tissue is only obtained from epithelial layers; also, lack of photons makes RS a much slower technology than IR [27]. Analysis of characteristic peaks and fingerprint regions confirms that decreased levels of glycogen and increased levels of DNA and amino acids in epithelial layers characterize cancerous cells [27]. In contrast to IR spectroscopy, RS has been applied for both in vivo and ex vivo measurements of biochemical constituents to identify dysplastic squamous tissue [29, 30]. Recent in vivo probes require few seconds for measuring a point spectrum and can be considered real-time [30]. A RS study of normal or HPV16-infected keratinocytes and a cervical cancer line was able to distinguish pairs of these lines with 70–100% sensitivity and 70–90% specificity [29]. The latest in vivo study showed that RS was superior to colposcopy regarding high-grade SILs (HGSILs) detection and the optimization of statistical algorithms for spectral variations may improve the classification performance [30].
Multi-spectral widefield imaging
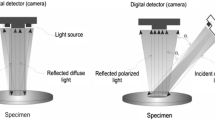
Multi-spectral widefield imaging enables real time, high resolution imaging of epithelial tissue combining autofluorescence and reflectance at multiple wavelengths across the entire cervical epithelium, videorate and color charge-coupled device [CCD] camera, easily available due to advances in computing and semiconductor technology [8]. Typically, widefield imaging can highlight suspicious regions of tissue with 50–100 μ spatial resolution and a single approach then can be used to interrogate suspicious areas with higher spatial or spectral resolution; however, true signal information is usually blurred due to contributions from out-of-focus components. Imaging spectroscopy combines conventional imaging with reflectance spectroscopy mainly with green wavelength illumination [31–33]. In contrast to conventional imaging where a macroscopic or microscopic scene is recorded and stored as a series of intensity values at each image element or pixel, the data usually take the form of a series of intensity values at various wavelengths [33]. Several studies have investigated features of multi-spectral imaging that could offer greater image contrast. For instance, when the incident light to the cervical tissue passes through a pair of polarizers with their polarization axes perpendicular to each other, regular reflectance component is reduced or eliminated and the recorded optical signal contains information only for the subsurface features, mainly for the subepithelial vessels [32]. Moreover, a multispectral reflectance study imaging the time course of acetowhitening demonstrated that the intensity of the backscattered light versus time was greater and persisted for longer time in HGSILs [33] (Fig. 2). Similarly, tissue autofluorescence particularly in the UV and blue wavelengths can be imaged in widefield mode offering a good discrimination between normal tissue and precancer [8].
Representative cases as displayed in the multispectral reflectance approach described by Orfanoudaki et al. [33], showing images of cervix after application of acetic acid (left column), expressed on a color scale (right column): normal (a); immature metaplasia (b); CIN I (c); CIN II (d); and CIN III (e)
A recent meta-analysis reported an unexpected overlap in the performance of point probe and multispectral devices though the observed heterogeneity among published studies regarding study design, selection criteria for recruited samples, classification system and disease threshold does not allow a precise comparison [34]. Also, the performance of these devices decreases as the sample size increases and clinical trial design progresses from pilot studies to Phase I to III trials [34]. Results of several large clinical trials investigating hyperspectral autofluorescence and reflectance imaging are summarized in Table 2. Ferris et al. [35] used a multimodal hyperspectral imaging device which combines tissue fluorescence at multiple excitation wavelengths with white light reflectance (SpectRx, Inc.) and noted a sensitivity of 97% and a specificity of 70% compared to colposcopic directed biopsy. In a prospective multicenter study of women who were scheduled to undergo colposcopy due to an abnormal Pap test or other risk factors, the sensitivity of spectroscopy was 95.1% with a corresponding 55.2% specificity for benign lesions [36]. Similarly, Huh et al. [37] used MediSpectra, a device which measures tissue fluorescence and white light backscattering, and concluded that spectroscopy using a multivariate classification algorithm could detect 33% more HGSILs than colposcopy alone with a relatively small increase in the false positive rate. A multicenter internally controlled trial evaluated the performance of a FDA-approved optical detection system named LUMA (Medispectra Inc., Lexington, MA) that combines native evoked fluorescence, diffuse reflectance backscatter, and video imaging, as an adjunct to colposcopy among women with abnormal cervical cytology [38]. The use of hyperspectal imaging resulted in a 21.2% relative gain in the true positive rate of colposcopy, yielding an incremental false positive rate of 18.1% [39]. A multi-center trial of this device involving 2,299 women randomized to receive colposcopy alone or colposcopy in conjunction with hyperspectral imaging for biopsy site selection showed similar results [40]. In addition, for women with an atypical Pap smear or low-grade SILs [LGSILs], the combined approach increased the true positive rate by 26.8% compared to colposcopy alone with a minimal increase in the false positive rate. Further large trials are required to evaluate the use of multispectral spectroscopy for the triage of patients with atypical Pap smears and its potential combined use with point optical spectroscopy and high resolution imaging.
Contrast agents for molecular imaging
Novel contrast agents attractive to use due to their optical properties and finite size may extend the in vivo diagnostic accuracy of optical methods. Optically active contrast agents consist of monoclonal antibodies or peptides against cancer biomarkers conjugated to an optically interrogatable label including metal nanoparticles, quantum dots and organic fluorescent dyes [41, 42]. Specifically, fluorescent dyes conjugated to monoclonal antibodies can image the distribution of multiple cell surface receptors over expressed on tumor cells, such as the epidermal growth factor receptor (EGFR) [42]. Other options include peptides such as the epidermal growth-factor that can target receptors, yielding smaller molecular-weight agents ideal for topical application and probes targeting protease activity in tumors [42]. Besides, a variety of semiconductor nanocrystals, named quantum dots, present many interesting properties including their luminescence. Quantum dots of different sizes that emit fluorescence at different wavelength can be excited at a single wavelength simultaneously providing a unique opportunity to do multi-color imaging experiments, however, concerns exist about their cytotoxicity [43].
Alternative contrast agents incorporate optically active, biocompatible metal nanoparticles as gold and silver bioconjugates which supply a strong source of backscattered light in widefield and high resolution imaging [42]. The enhancement of the electromagnetic field near the surface of the particles affects many optical phenomena including but not limited to Raman scattering and fluorescence intensity. Studies based on both cervical cancer lines and fresh cervical biopsies reported that gold nanoparticles increase the scattering cross section per particle when the particles agglutinate; indeed, the aggregation-induced increase in signal yields a contrast ratio of 10–20 fold between images of normal and HGSILs labeled with anti-EGFR gold nanoparticles [41]. The combination of gold nanoparticles or quantum dots with structure illumination microscopy or other technologies such as diffuse optical tomography that allow optical sectioning at a sub-cellular resolution may become the ideal modus operandi for the early precancer detection.
Confocal microscopy
Confocal microscopy (CM) enables exceptional high-resolution imaging at the cellular level at varying depths in the cervical epithelium, in histological detail. A confocal microscope is able to isolate light returning from the defined focal plane only, and to create a representative image of the reflectance values of each focal region after scanning it in axial and radial dimensions [44]. Reflectance CM allows the visualization of the increase in nuclear-to-cytoplasmic (N/C) ratio and nuclear density, while fluorescence mode allows mapping fluorophoric metabolites within subcellular structures [44]. While it is difficult to image weak autofluorescence in vivo using fluorescence CM due to photobleaching limits, advances in optically active contrast agents could further improve the diagnostic capability and reduce variations in image interpretation [45]. The application of acetic acid can enhance the nuclear signal in both normal and abnormal cervical biopsy specimens, seconds after its addition. In a pilot study of ex vivo cervical biopsies, CM detected the presence of HGSILs based on N/C ratio estimation with much higher sensitivity and specificity compared to colposcopy and the histological examination [46].
In vivo confocal endomicroscopy is a new technology that provides exceptional imaging of tissue microanatomy of cervical epithelium in near real time [45]. Confocal endomicroscopy could also be used to visualize dynamic changes in the microvasculature of the cervix in vivo and other features of the cervical epithelium including the squamo-columnar junction, dermal papillae and endocervical glands so as to differentiate tissue states such as inflammation, metaplasia and dysplasia [45]. However, the view field of these systems is limited, and it would be impractical to scan the surface of even a reasonably small organ like the uterine cervix.
Optical coherence tomography
Optical coherence tomography (OCT) is a non-invasive imaging technique that uses low coherence interferometry, to visualize the micro architecture of cervical epithelium for the identification of precancerous lesions [47]. Indeed OCT is able to image at depth within cervical tissue beyond the range of CM, and can yield back-scattering data for depths of 1-3 mm with a good image resolution, while CM images tissue within a few hundred microns of the surface [44]. OCT, first described in 1991, was firstly applied in ophthalmology and its working principle is similar to ultrasound pulse-echo imaging with the difference that OCT evaluates the optical rather than the acoustic reflectivity of the tissue exposed [48]. This novel imaging method has the potential to function as a real-time optical biopsy based on the imaging of the different optical scattering coefficients for epithelium and stroma and further analysis according to specific OCT imaging criteria that differentiate the patterns of normal form abnormal cervical microstructure of the various cervical anatomies [47, 49, 50]. OCT typically uses near infrared light sources in the range of 980–1,320 nm at harmless for the skin powers and the created contrast results from changes in the refractive index. Research systems today can measure 106 to 107 A-scans per second, which allows several volumes per second and so-called 4D OCT [51]. Issues with OCT are the amount of data generated which may create a need for computer-assisted data evaluation, limitations in backscattering contrast between normal and dysplastic tissue, the presence of inflammatory changes which could cause misinterpretation of OCT images, limited field of view, and limitations in imaging depth [51].
Regarding the clinical performance of OCT, its sensitivity and specificity when blinded to VIA and colposcopy results, were 56 and 59%, respectively [52]. Interestingly, an improved specificity was observed when OCT is used as adjunct to VIA followed by a significant loss in sensitivity or when used as an adjunct to the traditional management including colposcopy and biopsy; however, recent clinical trials recruiting large samples and using real-time OCT present conflicting results [52]. In particular, Wulan et al. [53], demonstrated that the addition of OCT to VIA increased the sensitivity for the detection of greater than or equal to CIN II from 43 to 62% with a parallel surprising loss in specificity from 96 to 80%. Gallwas et al. [49] was the only research group that reported high sensitivity (95%) but low specificity (45%) of OCT in the discrimination of CIN lesions. They provided as possible explanations the small sample size, inexperience in the interpretation of OCT images from inflammatory tissues and possible bias due to the fact that biopsies were taken only from suspicious areas. Prior to the validation of this new technology for the precancer detection, these trials should be repeated in different populations with large numbers of patients from both developed and developing countries.
Further refinement of this technology with the increase of both lateral and axial resolution and the use of fiberoptic probes with a disposable sheath, the establishment of strict diagnostic criteria that will form a computer algorithm, and the combined application with other imaging modalities will hopefully enhance the clinical performance of OCT. An interesting idea could be to combine OCT with fluorescence; until now, measurements for cervical tissue have not been published maybe due to technical challenges as OCT requires a focused beam, whereas fluorescence is designed to provide diffuse wide field illumination [54]. Also, a recent study presented a polarization-sensitive optical coherence tomography (PS-OCT) technique that quantifies the polarization changes induced by CIN and showed that quantifying the degree of circular polarization decay could discriminate CIN lesions [55]. Other potential uses of OCT include the imaging of the endocervical canal by a laser beam in 2-dimensions and the determination of the margins for a loop electro-excision procedure in a patient with a wide transformation zone though OCT readings obtained in this area could potentially contain angled images influencing the performance of the modality [52].
Conclusion
Hopefully, the application of the newly-developed non-invasive optical technology, both the spectroscopic techniques and the direct imaging methods, will offer a superior and not simply alternative option to current standard of care for the detection of cervical precancerous conditions. Optical technologies not only could serve as an “optical biopsy” to identify high-risk individuals who subsequently will be followed up closely but they may provide an automated, algorithm-based diagnosis at a single visit helping both developing countries where cost is a major deterrent to routine screening, and developed countries where over-treatment is a common observation (Fig. 3). Future refinements to the device ergonomics and optical resolution providing high quality optical image and automate image analysis coupled with the development of novel exogenous contrast agents could change clinical workflows in Gynecology. In parallel, the elucidation of the fundamental structural and biochemical origins of variations in remitted optical signals of normal and dysplastic tissue could facilitate the recognition and classification of intraepithelial lesions. Forthcoming methods of tissue-engineering promise to provide three-dimensional cell cultures that can help to understand the optical properties of human tissues in relation to biological events implicated in carcinogenesis. Multicenter randomized controlled trials are necessary to confirm the imaging potential of optical technology and to standardize discriminatory algorithms in order to determine their eventual implementation in primary screening of cervical cancer.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2002) Global cancer statistics. CA Cancer J Clin 55:74–108
Agosti JM, Goldie SJ (2007) Introducing HPV vaccine in developing countries–key challenges and issues. N Engl J Med 356:1908–1910
National Cancer Institute (NCI). Cervical Cancer Screening (PDQ®). Last Modified: 07/03/2010. http://www.cancer.gov/cancertopics/pdq/screening/cervical/healthprofessional
Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R (1998) Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 91:626–631
Sawaya GF, Brown AD, Washington AE, Garber AM (2001) Clinical practice. Current approaches to cervical-cancer screening. N Engl J Med 344:1603–1607
Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R (2011) Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet 113:14–24
Anonymous. Electro-optical sensors for the in vivo detection of cervical cancer and its precursors: submission guidance for an IDE/PMA. Rockville, MD. Food and drug administration. 1999 05/21/1999:Report No:266
Thekket N, Richards-Kortum R (2008) Optical imaging for cervical cancer detection: solutions for a continuing global problem. Nat Rev Cancer 8:725–731
Mourant JR, Canpolat M, Brocker C et al (2000) Light scattering from cells: the contribution of the nucleus and the effects of proliferative status. J Biomed Opt 5:131–137
Collier T, Follen M, Malpica A, Richards-Kortum R (2005) Sources of scattering in cervical tissue: determination of the scattering coefficient by confocal microscopy. Appl Opt 44:2072–2081
Drezek R, Guillaud M, Collier T et al (2003) Light scattering from cervical cells throughout neoplastic progression: influence of nuclear morphology, DNA content, and chromatin texture. J Biomed 8:7–16
Drezek R, Brookner C, Pavlova I et al (2001) Autofluorescence microscopy of fresh cervical-tissue sections reveals alterations in tissue biochemistry with dysplasia. Photochem Photobiol 73:636–641
Adrian Freeberg J, Benedet JL, West LA, Atkinson EN, MacAulay C, Follen M (2007) The clinical effectiveness of fluorescence and reflectance spectroscopy for the in vivo diagnosis of cervical neoplasia: An analysis by phase of trial design. Gynecol Oncol 107:270–280
Mourant JR, Bocklage TJ, Powers TM et al (2007) In vivo light scattering measurements for detection of precancerous conditions of the cervix. Gynecol Oncol 105:439–445
Chang SK, Mirabal YN, Atkinson EN et al (2005) Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt 10:024031
Georgakoudi I, Sheets EE, Muller MG et al (2002) Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo. Am J Obstet Gynecol 186:374–382
Nordstrom RJ, Burke L, Niloff JM, Myrtle JF (2001) Identification of cervical intraepithelial neoplasia (CIN) using UV-excited fluorescence and diffuse-reflectance tissue spectroscopy. Lasers Surg Med 29:118–127
Parker MF, Mooradian GC, Okimoto GS, O’ Connor DM, Miyazawa K, Saggese SJ (2002) Initial neural net construction for the detection of cervical intraepithelial neoplasia by fluorescence imaging. Am J Obstet Gynecol 187:398–402
Wright T, Ferenczy A, Wray S, Christinson R, Ganguly D (1999) Detection of cervical squamous intraepithelial lesions using evoked tissue fluorescence. Abstracts presented for the thirtieth annual meeting of the Society of gynecologic oncologists. Gynecol Oncol 72:45
Weingandt H, Stepp H, Baumgartner R, Diebold J, Xiang W, Hillemanns P (2002) Autofluorescence spectroscopy for the diagnosis of cervical intraepithelial neoplasia. Br J Obstet Gynecol 109:947–951
Chang SK, Follen M, Malpica A et al (2002) Optimal excitation wavelengths for discrimination of cervical neoplasia. IEEE Trans Biomed Eng 49:1102–1111
Mitchell MF, Cantor SB, Ramanujam N, Tortolero-Luna G, Richards-Kortum R (1999) Fluorescence spectroscopy for diagnosis of squamous intraepithelial lesions of the cervix. Obstet Gynecol 93:462–470
Mirabal YN, Chang SK, Atkinson EN, Malpica A, Follen M, Richards-Kortum R (2002) Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt 7:587–594
Steller W, Einenkel J, Horn LC et al (2006) Delimitation of squamous cell cervical carcinoma using infrared microspectroscopic imaging. Anal Bioanal Chem 384:145–154
Boydston-White S, Romeo M, Chernenko T, Regina A, Miljkoviζ M, Diem M (2006) Cell-cycle-dependent variations in FTIR micro-spectra of single proliferating HeLa cells: principal component and artificial neural network analysis. Biochim Biophys Acta 1758:908–914
Podshyvalov A, Sahu RK, Mark S et al (2005) Distinction of cervical cancer biopsies by use of infrared microspectroscopy and probabilistic neural networks. Appl Opt 44:3334–3725
Krishna CM, Prathima NB, Malini R et al (2006) Raman spectroscopy studies for diagnosis of cancers in human Uterine cervix. Vib Spectrosc 41:136–141
Mo J, Zheng W, Low JJ, Ng J, Ilancheran A, Huang Z (2009) High wavenumber Raman spectroscopy for in vivo detection of cervical dysplasia. Anal Chem 81:8908–8915
Jess PRT, Smith DDW, Mazilu M, Dholakia K, Riches AC, Herrington CS (2007) Early detection of cervical neoplasia by Raman spectroscopy. Int J Cancer 121:2723–2728
Robichaux-Viehoever A, Kanter E, Shappell H, Billheimer D, Jones H, Mahadevan-Jansen A (2007) Characterization of Raman spectra measured in vivo for the detection of cervical dysplasia. Appl Spectrosc 61:986–993
Balas CJ, Themelis GC, Prokopakis EP, Orfanoudaki I, Koumantakis E, Helidonis ES (1999) In vivo detection and staging of epithelial dysplasias and malignancies based on the quantitative assessment of acetic acid-tissue interaction kinetics. J Photochem Photobiol B 53:153–157
Balas C (2001) A novel optical imaging method for the early detection, quantitative grading, and mapping of cancerous and precancerous lesions of cervix. IEEE Trans Biomed Eng 48:96–104
Orfanoudaki I, Themelis G, Sifakis S et al (2005) A clinical study of optical biopsy of the uterine cervix using a multispectral imaging system. Gynecol Oncol 96:119–131
Adrian Freeberg J, Benedet JL, MacAulay C, West LA, Follen M (2007) The performance of fluorescence and reflectance spectroscopy for the in vivo diagnosis of cervical neoplasia; point probe versus multispectral approaches. Gynecol Oncol 107:248–255
Ferris DG, Lawhead RA, Dickman ED et al (2001) Multimodal multispectral Imaging for the noninvasive diagnosis of cervical neoplasia. J Low Genit Tract Dis 5:65–72
DeSantis T, Chakhtoura N, Twiggs L et al (2007) Spectroscopic imaging as a triage test for cervical disease: a prospective multicenter clinical trial. J Low Genit Tract Dis 11:18–24
Huh WK, Cestero RM, Garcia FA et al (2004) Optical detection of high-grade cervical intraepithelial neoplasia in vivo: results of a 604-patient study. Am J Obstet Gynecol 190:1249–1257
Kendrick JE, Huh WK, Alvarez RD (2007) LUMA cervical imaging system. Expert Rev Med Devices 4:121–129
Alvarez RD, Wright TC (2007) Increased detection of high-grade cervical intraepithelial neoplasia utilizing an optical detection system as an adjunct to colposcopy. Gynecol Oncol 106:23–28
Alvarez RD, Wright TC (2007) Effective cervical neoplasia detection with a novel optical detection system: a randomized trial. Gynecol Oncol 104:281–289
Adams KE, Ke S, Kwon S et al (2007) Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt 12:024017
Aaron J, Nitin N, Travis K et al (2007) Plasmon resonance coupling of metal nanoparticles for molecular imaging of carcinogenesis in vivo. J Biomed Opt 12:034007
Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R (2006) Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small 2:1412–1417
Drezek R, Richards-Kortum R, Brewer MA et al (2003) Optical imaging of the cervix. Cancer 98:2015–2027
Tan J, Quinn M, Pyman J, Delaney P, McLaren W (2009) Detection of cervical intraepithelial neoplasia in vivo using confocal endomicroscopy. BJOG 116:1663–1670
Collier T, Lacy A, Richards-Kortum R, Malpica A, Follen M (2002) Near real-time confocal microscopy of amelanotic tissue: detection of dysplasia in ex vivo cervical tissue. Acad Radiol 9:504–512
Pitris C, Goodman A, Boppart SA, Libus JJ, Fujimoto JG, Brezinski ME (1999) High-resolution imaging of gynecologic neoplasms using optical coherence tomography. Obstet Gynecol 93:135–139
Huang D, Swanson EA, Lin CP et al (1991) Optical coherence tomography. Science 254(5035):1178–1181
Gallwas J, Turk L, Friese K, Dannecker C (2010) Optical coherence tomography as a non-invasive imaging technique for preinvasive and invasive neoplasia of the uterine cervix. Ultrasound Obstet Gynecol 36:624–629
Escobar PF, Belinson JL, White A et al (2004) Diagnostic efficacy of optical coherence tomography in the management of preinvasive and invasive cancer of uterine cervix and vulva. Int J Gynecol Cancer 14:470–474
Fujimoto JG, Pitris C, Boppart SA, Brezinski ME (2000) Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2:9–25
Escobar PF, Rojas-Espaillat L, Tisci S et al (2006) Optical coherence tomography as a diagnostic aid to visual inspection and colposcopy for preinvasive and invasive cancer of the uterine cervix. Int J Gynecol Cancer 16:1815–1822
Wulan N, Rasool N, Belinson SE et al (2010) Study of the diagnostic efficacy of real-time optical coherence tomography as an adjunct to unaided visual inspection with acetic acid for the diagnosis of preinvasive and invasive neoplasia of the uterine cervix. Int J Gynecol Cancer 20:422–427
Barton JK, Guzman F, Tumlinson A (2004) Dual modality instrument for simultaneous optical coherence tomography imaging and fluorescence spectroscopy. J Biomed Opt 9:618–623
Lee SW, Yoo JY, Kang JH et al (2008) Optical diagnosis of cervical intraepithelial neoplasia (CIN) using polarization-sensitive optical coherence tomography. Optics Express 2709–2719
Liu Z, Belinson SE, Li J et al (2010) Diagnostic efficacy of real-time optical coherence tomography in the management of preinvasive and invasive neoplasia of the uterine cervix. Int J Gynecol Cancer 20:283–287
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orfanoudaki, I.M., Kappou, D. & Sifakis, S. Recent advances in optical imaging for cervical cancer detection. Arch Gynecol Obstet 284, 1197–1208 (2011). https://doi.org/10.1007/s00404-011-2009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-011-2009-4