Abstract
Objectives
Rosiglitazone, an insulin sensitizing agent is used currently in women with clomiphene citrate (CC) resistant polycystic ovarian syndrome (PCOS). Our study proposed to compare the efficacy of rosiglitazone and CC with laparoscopic ovarian drilling (LOD) and CC in terms of biochemical effects, ovulation rate and pregnancy rate in patients of PCOS resistant to CC.
Methods
This prospective randomised trial included 43 patients of PCOS resistant to CC. Twenty-two women were assigned to the rosiglitazone (4 mg twice daily) and CC group and other 21 patients underwent unilateral LOD and then received CC and multivitamins. The treatment continued for six cycles in both the groups. The biochemical response, ovulation rate and pregnancy rate over a follow up period of 6 months were compared.
Results
Treatment with rosiglitazone and CC or LOD and CC resulted in increased ovulation (80.8 vs. 81.5%) and pregnancy (50 vs. 42.8%), respectively. There was no statistical difference between the two groups in terms of biochemical response, ovulation rate and pregnancy rate.
Conclusion
To avoid the risk of adverse effects of LOD preference may be given to the use of rosiglitazone and CC therapy in patients of PCOS resistant to CC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovarian syndrome (PCOS) is a common endocrinopathy among women of reproductive age [1, 2]. It is frequently associated with insulin resistance and compensatory hyperinsulinemia. Clomiphene citrate (CC) is currently the first line therapeutic modality for women with infertility and PCOS. PCOS patients are frequently resistant to CC and these results in numerous cycles where CC is unsuccessfully used for ovulation induction. The use of novel insulin sensitizers such as biguanides and thiazolidenediones promise a new treatment options for PCOS [3]. Addition of metformin in the CC resistant patients is highly effective in achieving ovulation induction [4]. But metformin is associated with gastrointestinal side effects. The newer thiazolidenedione rosiglitazone is alternative to metformin in CC resistant PCOS with lesser side effects [5]. Laparoscopic ovarian drilling (LOD) is another effective option in PCOS resistant to CC though there is concern about postoperative adhesion and theoretical long-term risk of premature ovarian failure (POF) [6, 7].
Various studies reported till date has compared either metformin with rosiglitazone or metformin with LOD [5, 8]. There are no studies available comparing rosiglitazone with LOD in CC resistant PCOS. This present study was conducted to evaluate and compare the efficacy of rosiglitazone and CC with LOD + CC in women with CC resistant PCOS.
Materials and methods
The women were recruited from the gynaecological clinic of our hospitals from January 2006 to 2009. The necessary ethical clearance by the institutional review board was taken prior to commencing the study. The inclusion criteria included patients in the age group 20–40 years, having primary infertility with CC resistant PCOS, had documented patent tubes on hystero-salpingography and no other infertility factor. PCOS patients who failed to ovulate with a dose of CC of 150 mg/day for 5 days from day 3 of the menstrual cycle were considered as CC resistant PCOS. The patients of PCOS were recruited in the presence of oligomenorrhoea (i.e. interval between periods was ≥35 days) or amenorrhoea (i.e. absence of vaginal bleeding for 6 months), hirsutism, enlarged ovaries with multiple follicles (≥10 measuring 2–8 mm in diameter) arranged peripherally on transvaginal ultrasonography (USG), and/or elevated serum testosterone. Finally the diagnosis of PCOS was made on the basis of revised Rotterdam 2003 criteria [9]. Presence of 2 out of 3 criteria (Oligo and/or anovulation, clinical biochemical sign of hyperandogenism and polycystic ovaries) was recommended as a diagnostic of PCOS. All women had normal hysterosalpingography and their partners had normal semen parameters. The primary exclusion criteria were other PCOS like syndromes, including Cushing’s syndrome, congenital adrenal hyperplasia, androgen producing tumours, hyperprolactinemia and hypothyroidism. All women were evaluated by a detailed history and clinical examination. The anthropomentric measurements included height, weight, body mass index (BMI) and waist-to-hip ratio (WHR). BMI was measured as the ratio between the weight and square of the height (kg/m2). WHR was calculated as the ratio between the smallest circumference of torso (between the 12th rile and the iliac crest) and circumference of the hip (considered as the maximal extension of the buttocks). The same operator, who was blinded to the treatment allocation, performed all measurements. Patients were evaluated on the first day of spontaneous cycle or withdrawal bleeding after a 5-day course of 10 mg/day medroxyprogesterone acetate (MPA). Venous blood was collected between 8 a.m. to 10 a.m. after an overnight fast and the following hormones were measured: follicle stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSH), free thyroxin (T4), total testosterone (T), free T, dehydroepiandrosterone sulphate (DHEA-S), 17-OH-Progesterone (17-OH-P), glucose and serum insulin. The glucose (nmol/L)-to-insulin (pmol/L) ratio (GIR) proposed as an index of peripheral insulin sensitivity [10] was also calculated. During the same visit all patients underwent a baseline transvaginal USG. The patients were randomly allocated into two treatment groups of 25 women each (group A and group B). Randomization was carried out using online software (http://www.randomization) to generate a random number table. The randomization was completed by opening sealed envelopes containing numbers from the computer generated random table. All patients underwent laparoscopy and patients who had other factors found on laparoscopy which can be responsible for infertility, were excluded from the study. Unilateral LOD was done only in group B patients by same experienced surgeon and number of drill was 5. The surface of the ovary was cooled by irrigating with normal saline solution and 300–500 mL normal saline was left in the pelvis to prevent adhesion. After laparoscopy patients in group A were treated with Rosiglitazone (Rezult, Sun pharmaceutical industries, Jammu, India) at a dose of 4 mg twice daily and CC (Siphene, Serum Institute of India, Pune, India) at a dose of 100 mg daily from the third day of the period for 5 days. A baseline liver function test was performed in these patients before starting rosiglitazone. In the presence of abnormal liver function test, the patients were excluded from the study. The patients in group B received multivitamins (two tablets daily orally) and CC (100 mg daily) from the third day of the period for 5 days. Multivitamins were added to CC in patients of group B as placebo. Duration of treatment in both the groups was 6 months. In both the groups, ovulation was assessed by serial USG at mid-cycle until visualization of pre-ovulatory follicle of at least 18 mm. Ovulation was confirmed by seeing follicle collapse on subsequent USG and elevated serum progesterone level (≥25 nmol/L). Each woman was asked to have timed intercourse after ovulatory dose of HCG (5,000–10,000 IU). In group A once the patient misses her period and urine pregnancy test was positive, rosiglitazone was discontinued and ultrasound was arranged to document pregnancy. Women in both the groups without evidence of ovulation and with negative pregnancy tests were asked to follow the respective schedule of treatment in subsequent cycles. Clinical and biochemical monitoring of any adverse drug reactions was done during and at the end of each treatment cycle. Hormone tests performed at the baseline was repeated on the first day of menses in women who did not become pregnant after three cycles and six cycles of rosiglitazone and CC or LOD and CC.
The variables which were taken as outcome of this study included ovulation rate, number of follicles and serum estradiol (E2) on day 12 of the cycle, pregnancy rate, changes in the fasting levels of plasma glucose and other endocrine parameters including serum insulin, total T, free T, FSH, LH, DHEAS, 17-OHP and GIR. Results were presented as mean ± SD and data were analyzed using SPSS, Statistical Package, version 11.0 for windows. Qualitative data were analyzed using ‘Chi’ square test or Fischer’s exact test as applicable and the quantitative data were analyzed using the Student’s t test. Comparison of values of various parameters taken before and after treatment was made by using the student two-tailed, unpaired t test and a p value < 0.05 was considered as significant.
Result
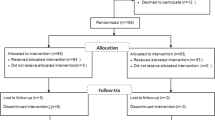
During the study period a total number of 50 women were recruited for the study and 5 patients lost follow-up. Out of 45 women 2 women refused to participate in the study before randomization and therefore 43 women entered and completed the study. Twenty-two patients in group A were assigned to the rosiglitazone and CC group and the rest 21 patients were assigned to LOD and CC group. All these 43 patients were available for follow up and were included for analysis. The women studied in both the groups were young [27.32 ± 4.25, 23–33 years (mean ± SD, range) in the rosiglitazone and CC group and 28.42 ± 3.65, 23–34 years in LOD and CC group] and there was no statistical difference in age when compared in both groups. In both the groups the patients were not obese with BMI and WHR <30 and <0.8, respectively. Before treatment 30 patients had oligomenorrhoea and 13 patients were amenorrhoeic. No significant differences were found in the other base line characteristics of both groups (Table 1). The ovulation rate in rosiglitazone + CC group and LOD + CC group was similar (19 of 22 (86.3%) [72 out of 89 cycles (80.8%)] in rosiglitazone + CC versus (17 of 21 (80.9%) [72 out of 92 cycles (78.2%)] in LOD + CC group). Similarly no statistical differences were found in the number of follicles ≥ 14 mm in the rosiglitazone + CC group (2.4 ± 1) (mean ± SD) compared to LOD + CC group (2.2 ± 1) (p = 0.68) and E2 on day 12 in the rosiglitazone + CC group (968 ± 426 pmol/L) compared to LOD + CC group (1,024 ± 325 pmol/L) (p = 0.72). There was no occurrence of ovarian hyperstimulation syndrome (OHSS) in both the groups. There was also no statistical difference in the pregnancy rate when both groups were compared [11 of 22 (50%) in rosiglitazone + CC group versus 9 of 22 (42.8%) in LOD + CC group] (p = 0.59). The pregnancy rate per cycle was 12.3% for rosiglitazone + CC group and 9.2% for LOD + CC group. The cumulative pregnancy rate was similar in both the group as depicted in Figs. 1 and 2. There was one twin pregnancy in rosiglitazone + CC group and two twin pregnancies in LOD + CC group. There was one missed abortion in each group. There are nine ongoing pregnancies and the rest of the pregnant women completed their pregnancies with successful live births. The various endocrine and metabolic parameters of women with PCOS before and after treatment with rosiglitazone + CC or LOD + CC are depicted in Table 2.
Both the rosiglitazone + CC and LOD + CC groups show no significant changes in fasting plasma glucose. The fasting serum insulin decreased significantly by 41.5% in the rosiglitazone + CC group (p = 0.04) compared to in LOD + CC group where the fasting insulin decreased by only 12.5%. This was accompanied by significant increase in the GIR values in rosiglitazone + CC group compared to LOD + CC group (65.1 vs. 14% respectively). Although both rosiglitazone and CC and LOD + CC groups exhibited a significant decline in the levels of LH (38.5 vs. 28.5% respectively), no significant differences were observed in the FSH values. However there were significant decreases in the level of total T (49 vs. 43.2%), free T (40.3 vs. 43.1%) and DHFAS (34.8 vs. 32.6%) in the rosiglitazone + CC and LOD + CC groups, respectively. There were no major side effects observed with the use of rosiglitazone.
Discussion
Polycystic ovarian syndrome is the most common cause of anovulatory infertility and is responsible for 70% of infertility due to anovulation [11]. About 10–30% of these patients will remain anovulatory after 6 months of treatment with CC [12]. In women with CC resistant PCOS, the use of novel insulin sensitizers such as biguanides and thiazolidenediones promise new treatment options [3, 5]. LOD has also been widely established as an effective second line method of induction in CC resistant PCOS patients with high ovulation (80%) and pregnancy rate (60–80%) [7, 13, 14]. Ghazeeri et al. [15] treated 25 patients with rosiglitazone and CC (13 women) or rosiglitazone and placebo (12 women) for 2 months. This study reported that the ovulation rate in women treated with rosiglitazone and CC was 77% compared with 33% in women with rosiglitazone only. Belli et al. [16] demonstrated that treatment with rosiglitazone decreased LH levels, improved insulin resistance (IR) parameters and normalized menstrual cycles in women with PCOS and IR. Another study [17] reported that out of 12 obese patients with PCOS with severe IR who received 6 months rosiglitazone therapy, 11 (91%) women reverted to regular ovulatory cycling during the treatment period. More recently, Rouzi et al. [5] showed insulin-sensitizing therapy with rosiglitazone and CC or metformin and CC resulted in increased rate of ovulation (64.3 vs. 36.4%) and pregnancy (58.3 vs. 38.5%), respectively, in CC resistant women with PCOS. They concluded in this study that short-term use of rosiglitazone and CC is more efficacious than metformin and CC in ovulation induction in women with CC resistant PCOS. Another important study by Lergo et al. [18] did not find a significant benefit of combination therapy with CC and metformin over CC alone in patients of PCOS. Regarding LOD, though various studies [19, 20] have shown encouraging results in CC resistant PCOS, there is an ongoing concern about the adverse effects of LOD particularly on periovarian adhesion formation and ovarian function [21, 22]. Recent Cochrane review 2008 [23] suggests that post operative adhesion formation and theoretical long-term risk of POF after LOD should be kept in mind. In this regard our previous study [24] and other recent studies [25] suggested that unilateral LOD may be a suitable option in CC resistant PCOS which can replace bilateral LOD with a potential advantage of decreasing the chances of adhesion formation. It has been reported that patients of PCOS resistant to CC respond once again to CC treatment after LOD [14]. Based on these supportive evidences we did unilateral ovarian drilling in patient of LOD and added CC after LOD in LOD + CC group. To the best of our knowledge there is no randomized trial available in the literature comparing the clinical and endocrinological outcome with rosiglitazone versus LOD in patient of CC resistant PCOS. In the present study, treatment with rosiglitazone and CC or LOD + CC resulted in increased ovulation (80.8 vs. 81.5%) and pregnancy (50 vs. 42.8%), respectively, in CC resistant women with PCOS. The outcome in terms of ovulation rate and pregnancy rate in both the groups was not statistically significant. Similarly no statistical difference was found in both the groups in terms of number of follicle ≥14 mm and serum E2 level on day 12 of the cycle. Ghazeeri et al. [15] demonstrated significant decrease in the serum levels of LH, total T and DHEAS in CC resistant women with PCOS treated with rosiglitazone. The changes in LH levels were different from that described in previous studies in women with PCOS treated with rosiglitazone, which showed decrease in circulating androgen but without any changes in LH values [26, 27]. In our study the rosiglitazone and CC group showed significant decrease in the serum levels of LH, total T and DHEAS. Similar results were also obtained in LOD + CC group.
It has been reported that with rosiglitazone therapy, there was an improvement in IR, as indicated by the changes in the values of GIR [5]. In our study we found there is significant decrease in serum fasting insulin and GIR values in rosiglitazone group (p < 0.04) whereas the decrease in insulin and GIR values was not statistically significant in LOD + CC group. The normalization of insulin levels in response to therapy with different insulin sensitizing drugs including rosiglitazone, metformin, and others in women with PCOS suggests that the metabolic and reproductive abnormalities might be related to the hyper insulinemia per se and not to any specific mechanism of IR [5]. Rosiglitazone decreases hepatic fat content and increases insulin sensitibility in muscles and thereby making the drug more useful in patients with insulin resistance [5]. On the other hand in LOD, the hypothesis given is that the response of the ovary to injury leads to a local cascade of growth factors and such as insulin like growth factor I which interact with FSH [28]. This results in stimulation of follicular growth and the production of hormone gonadotropin surge attenuating/inhibitory factor, which leads to fall in serum LH concentration [29]. Adverse effects reported with rosiglitazone therapy include peripheral oedema, fall in hemoglobin and hematocrit [5]. In our study we did not find any such side effects with rosiglitazone therapy. Since our sample size was small, we do acknowledge that there is a possibility of type II (beta) error in this study. This may be addressed by recruiting more number of patients or applying non-parametric tests in future randomized control trials.
Conclusion
It appears that rosiglitazone and CC therapy is equally efficacious in terms of ovulation and pregnancy rate when compared with therapy with LOD + CC in patient of CC resistant PCOS. Since there is an ongoing concern of adverse effects with LOD, preference may be given to the use of rosiglitazone and CC therapy as a promising therapeutic option in these patients.
References
Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC (2006) Endocrine and metabolic effects of rosiglitazone in overweight women with PCOS: a randomized placebo-controlled study. Hum Reprod 21(6):1400–1407
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (2006) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749
The practice Committee of the American Society for Reproductive Medicine (2004) Use of insulin sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril 82:S181–S183
Siebert TI, Kruger TF, Steyn DW, Nosarka S (2006) Is the addition of metformin efficacious in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome? A structured literature review. Fertil Steril 86(5):1432–1436
Rouzi AA, Ardawi MSM (2006) A randomized controlled trial of the efficacy of rosiglitazone and clomiphene citrate versus metformin and clomiphene citrate in women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 85(2):428–435
Amer SA, Li TC, Metwally M, Emarh M, Ledger WL (2009) Randomized controlled trial comparing laparoscopic ovarian diathermy with clomiphene citrate as a first-line method of ovulation induction in women with polycystic ovary syndrome. Hum Reprod 24(1):219–225
Farquhar C, Lilford R, Marjoribanks J, Vanderkerckhove P (2005) Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev 18(3):CD001122
Malkawi HY, Qublan HS, Hamaideh AH (2003) Medical vs. surgical treatment for clomiphene citrate-resistant women with polycystic ovary syndrome. J Obstet Gynaecol 23:289–293
The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group, Fauser BCJM (2004) Revised 2003 consensus on diagnostic criteria and long-term health risk related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1):41–47
Legros RS, Finegood D, Dunaif A (1998) A fasting glucose-to-insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 83:2694–2698
Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N (2002) Ovarian Function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab 87:569–574
Hughes E, Collins J, Vandekerckhove P (2000) Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst Rev 2:CD000056 (Cochrane Review)
Amar NA, Lahelin GCL (1993) Laparoscopic ovarian diathermy: an effective treatment for anti-oestrogen resistant anovulatory infertility in women with polycystic ovary syndrome. Br J Obstet Gynaecol 100:161–164
Bayram N, Van Wely M, Kaaijk E, Bossuyt P, Van Der Veen F (2004) Using an electrocautery strategy of recombinant follicle stimulating hormone to induce ovulation in polycystic ovary syndrome: randomized controlled trial. Br Med J 328:192–196
Ghazeeri G, Kutteh WH, Byer-Ash M, Haas D, Ke RW (2003) Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril 79:562–566
Belli SH, Graffigna MN, Oneto A, Otero P, Schurman L, Levalle O (2004) Effect of rosiglitazone on insulin resistance, growth factors, and reproductive disturbances in women with polycystic ovary syndrome. Fertil Steril 81:624–629
Sepillian V, Nagamani M (2005) Effects of rosiglitazone in obese women with polycystic ovary syndrome and severe insulin resistance. J Clin Endocrinol Metab 90:60–65
Legro RS, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP et al (2007) Clomiphene, Metformin, or Both for infertility in the polycystic ovary syndrome. N Engl J Med 356(6):551–556
Gjonnaess H (1984) Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertil Steril 41:20–25
Yousef H, Atallah MM (2007) Unilateral ovarian drilling in polycystic ovarian syndrome: a prospective randomized study. Reprod Biomed Online 15(4):457–462
Gurgan T, Urman B et al (1992) The effect of short interval laparoscopic lysis of adhesions in pregnancy rates following Nd: YAG laser photocoagulation of PCO. Obstet Gynecol 80:45–47
Rajashekar L, Krishna D, Patil M (2008) Polycystic ovaries and infertility: our experience. J Hum Reprod Sci 1(2):65–72
Farquhar C, Lilford RJ, Marjoribanks J Vandekerckhove P (2008) Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochran Database Syst Rev 4:CD00122
Roy KK, Jinee B, Nidhi M, Sunesh K (2009) Evaluation of unilateral versus bilateral ovarian drilling in clomiphene citrate resistant cases of polycystic ovarian syndrome. Arch Gynecol Obstet 279:401–402
Al-Mizyen E (2007) Unilateral laparoscopic ovarian diathermy in infertile women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 88(6):1678–1680
Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R (1996) The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab 81:3299–3306
Azziz R, Ehrmann D, Legro D, Whitcomb RW, Hanley R, Gmerek-Fereshetian A, PCOS/Troglitazone Study Group (2001) Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blinded, placebo-controlled trial. J Clin Endocrinol Metab 86:1626–1632
Adashi EY, Resnick CE, Hernandez ER (1998) Insulin-like growth factor 1 as an amplifier of FSH: studies on mechanism(s) and site(s) of action in cultured rat granulosa cells. Endocrinol 122:1583–1591
Balen AH, Jacobs HS (1991) Gonadotrophin surge attenuating factor: a missing link in the control of LH secretion? Clin Endocrinol 35:399–402
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, K.K., Baruah, J., Sharma, A. et al. A prospective randomized trial comparing the clinical and endocrinological outcome with rosiglitazone versus laparoscopic ovarian drilling in patients with polycystic ovarian disease resistant to ovulation induction with clomiphene citrate. Arch Gynecol Obstet 281, 939–944 (2010). https://doi.org/10.1007/s00404-009-1305-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-009-1305-8