Abstract
Purpose
The aim of this study was to assess the effect of vitamin D supplementation on ovulation rate in overweight subfertile women with PCOS undergoing ovulation induction.
Methods
This was a single center, parallel-groups, double-blind, and placebo-controlled randomized trial involving 186 eligible women undergoing induction of ovulation with clomiphene citrate (Clomid®, Aventis) 50 mg tablet twice daily starting from the third day of menstrual cycle and for 5 days combined with either oral Vitamin D (ossofortin®, EVA PHARMA) 10,000 IU twice weekly and calcium (calciprex®, Marcyrl Pharmaceutical Industries) 1250 mg twice daily or to receive a placebo with calcium for three successive induction cycles. The vitamin D or placebo supplementation started 1 month before induction cycles (total four cycles). Cycles were monitored with ultrasound follicle tracking and mid-luteal serum progesterone measurement. The primary outcome was the ovulation rate after three induction cycles.
Results
The study was performed during the period between January 2018 and September 2018, Eighty six (92.5%) women in the treatment group and 73 (78.5%) in the control group had successful ovulation (p = 0.007). The absolute and relative risk reduction was 14% and 65% respectively. Biochemical and clinical pregnancy occurred in 61.3 and 50.5% in the treatment group, and in 49.5 and 39.8% in the control group (p = 0.105 and 0.141 respectively).
Conclusion
In subfertile women with PCOS undergoing induction of ovulation, vitamin D supplementation significantly improved the ovulation rate; however, there was no effect on clinical or biochemical pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovarian syndrome (PCOS) is a common condition with a variable range of reproductive and metabolic features that affects 4–18% women during their reproductive years [1] and accounts for 90–95% of anovulatory subfertile women seeking fertility [2].
Vitamin D (VD) has received universal attention because of its many health benefits. Despite the general agreement about its major role in bone health, there remains skepticism about the nonskeletal health benefits of VD [3]. Recently, a large meta-analysis analyzing the findings of 81 randomized trials, and involving more than 50,000 participants declared that VD supplementation does not prevent fractures or falls, or have clinically meaningful effects on bone mineral density raising doubts regarding its musculoskeletal benefits [4].
VD deficiency has become an endemic problem in many countries, it is the most common nutritional deficiency worldwide [5] In contrast to a prevalence of 20–48% among the general adult population [6], many studies reported that the prevalence of VD deficiency in women with PCOS approached more than 80 percent [1, 7, 8]. Moreover, it is more prevalent among women with PCOS with elevated body mass index (BMI), as VD is deposited in adipose tissues, making it unavailable for the body to use [9, 10]. In Egypt, VD deficiency was found in 72.6% of the nursing women, 54% of the pregnant women, 72% of the females in childbearing period, 39.5% of the elderly group, and 77.2% of the geriatric group [11]. VD deficiency is a major contributing factor to insulin resistance, obesity, metabolic syndrome (MS), and ovulatory dysfunction, all of which are commonly observed in PCOS [12]. Many recent studies [8, 13, 14] reported a relation between low serum level of VD and ovulatory disorders observed in PCOS with possible beneficial effects of VD supplementation on ovarian follicles maturation, ovulation, and menstrual regularity. Moreover, VD deficiency, through calcium dysregulation, can contribute to follicular arrest [15]. It has been suggested that serum calcium levels may play a possible role in follicle selection, as calcium receptors have been found to be expressed in preovulatory granulosa explants [16].
In a study by Firouzabadi et al. [8], there was no statistically significant difference between VD and the placebo regarding menstrual regularity (70 vs. 58%, p = 0.211), and follicle maturation (28 vs. 22%, p = 0.698). According to the data regarding pregnancy in PCOS II RCT, Butts et al. reported that subjects with VD deficiency were less likely to ovulate (AOR 0.82, 95% CI 0.68–0.99, p = 0.04) with a 40% lower chance of LB (AOR 0.63, 95% CI 0.41–0.98, p = 0.04) than those with sufficient VD [17].
Two systematic reviews [1, 12] looked at the role of VD on follicular development and linked ovulatory dysfunction in women with PCOS with its deficiency. They raised the attention to the need for randomized trials to elucidate the impact of VD supplementation on ovulatory dysfunction associated with PCOS. Luk et al. [18], suggested that owing to its accessibility, ease of administration and wide range of safety; as, exogenous VD intoxication is usually caused by prolonged use (months) of VD mega doses, VD supplementation may be a cost effective strategy to improve the reproductive health.
The unsolved doubts regarding the role of VD in the pathophysiology of many chronic diseases, the validity of the basis of diagnosing its deficiency, and the effect of correcting of the deficiency state on preventing adverse health consequences are raised and are the key questions to many ongoing researches [19, 20].
The aim of this study was to evaluate the effect of VD and calcium supplementation compared with placebo and calcium supplementation on the ovulation rate in overweight anovulatory subfertile women with PCOS undergoing induction of ovulation.
Materials and methods
Study population
This study was a single center, parallel-groups, double-blind, and placebo-controlled randomized clinical that was conducted on 186 eligible women undergoing induction of ovulation at Ain Shams University Maternity Hospital infertility outpatient clinic during the period between January 2018 and September 2018.
The study population was a consecutive series of overweight (body mass index (BMI) between 25 and 29.9 kg/ht2)subfertile women (age between 25 and 35 years), with PCOS (diagnosed by using ESHRE/ASRM criteria) [21]. All participants had at least two of the three criteria: (1) ovulatory dysfunction (oligo-/anovulation), (2) hyperandrogenism (defined by the clinical presence of hirsutism (the modified Ferriman–Gallwey score ≥8), acne or alopecia, and/or elevated testosterone level ≥2.5 nmol/l), and/or (3) ultrasound finding of polycystic ovaries (defined by an increased number of small antral follicles ≥12 follicles (2–9 mm) and/or an ovarian volume of >10 cm3 in one or both ovaries that was performed by the principal investigator (Y.S.) to avoid inter observer variability. Other disorders that mimic PCOS, including thyroid disease and prolactin excess, adult onset adrenal hyperplasia were ruled out. All participants had proven at least one fallopian tube patency and normal semen analysis of their male partners.
We excluded subfertile women due to causes other than PCOS, women with history of 6 months or less from previous treatment with any of the sex steroids, infertility drugs, insulin sensitizers, or any other hormonal treatment and women with hypersensitivity to any medication was used in the study.
Any woman eligible to participate was informed about the trial by one of the researchers. Those approved to participate gave informed written consent.
One hundred and eighty-six consecutive women were randomly allocated to receive clomiphene citrate for induction of ovulation in addition to VD and calcium supplementation (intervention group) or received clomiphene citrate for induction of ovulation with placebo and calcium supplementation (control group), for the same duration as the intervention group.
Allocation to either one of the two groups was in a 1:1 ratio. Randomization numbers were completed by using the computer-generated list of random numbers. An independent statistician not involved in the treatment or data collection was responsible for random allocation of participants to the two groups. The final group assignment was sealed in sequentially numbered opaque envelopes and the data collector, and the participants were blinded in this trial as both drug with active ingredient and placebo were provided in identical sealed opaque containers, equal in weight, similar in appearance, and tamper proof.
All participants were subjected to a detailed clinical assessment including a detailed history, general, abdominal, pelvic examinations, pelvic ultrasound, hormonal assay, and oral glucose tolerance test. (Blood samples were collected after an overnight 8 h fast with a stable diet for the preceding 72 h and in the early follicular phase (day 3) of the spontaneous menstrual cycle in regularly menstruating women or any day in amenorrheic women.
Blood samples were aliquoted for follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), prolactin, thyroid-stimulating hormone (TSH), total testosterone, and glucose tolerance. After obtaining the fasting blood sample, an oral glucose tolerance was performed with 75 gm anhydrous glucose dissolved in 250–300 mL of water and blood samples were collected at 120 min for plasma glucose. The blood samples were centrifuged and analyzed using enzyme linked immunosorbent assay (ELISA) (Advia-Centaur; XP, France).
We did not measure VD levels at the baseline because measuring its level is expensive and it would raise ethical concerns weather deficient women should be instructed to receive the supplementation before induction, meanwhile many empirical treatments were used as an adjuvant therapy in anovulatory women using clomiphene citrate for OI, we thought that empirical use of VD would be safe, widely available, and easily accessible.
The VD and calcium supplementation or placebo and calcium were started 1 month before the first induction cycle and continued throughout the induction cycles.
Participants with oligo/anovulation received two tablets of norethisterone 5 mg tab (Steronate nor®, hi pharm) every 12 h for 5 days to allow withdrawal bleeding before start ovulation induction (OI) by clomiphene citrate (Clomid®, Aventis) 50 mg tablet twice daily 12 h apart (total dose 100 mg daily) starting from the third day of menstrual cycle and for 5 days.
The intervention group received: VD (ossofortin®, EVA PHARMA) 10,000 IU twice weekly [22] and calcium (calciprex®, Marcyrl Pharmaceutical Industries) 1250 mg twice daily which provide elemental calcium 500 mg/tablet for 1 month before induction of ovulation and both were continued during the three induction cycles in the same regimen. While the control group received: a placebo tablet twice weekly and calcium (calciprex®, Marcyrl Pharmaceutical Industries) 1250 mg twice daily which provide elemental calcium 500 mg/tablet for 1 month before induction of ovulation and both were continued during the three consecutive induction cycles in the same regimen.
Serial transvaginal ultrasound was performed using VSON (E6) transvaginal probe frequency 4–9 MHz to monitor the number and size of developing follicles to time human chorionic gonadotropin (hCG) administration (Choriomon® IBSA) 5000 IU ampoule, single dose 10,000 IU intramuscular which was given when the leading follicle reaches 18–20 mm in diameter to induce ovulation. All participants were advised about timed intercourse during the treatment cycles.
The primary outcome was the rate of ovulation. It was monitored for three successive induction cycles after starting CC if pregnancy did not occur.
The secondary outcomes were pregnancy rate (both clinical and biochemical), endometrial thickness (ET) (at the time of hCG injection and mid luteal), and the rate of adverse effects such as hot flashes headache, nausea, vomiting, breast tenderness, blurred vision, abdominal distention/pain, and ovarian enlargement or hyperstimulation. (These outcomes were assessed at the time of hCG injection, mid luteal, and at the early follicular phase of the subsequent induction cycle).
Ovulation was diagnosed with a progesterone level of ≥19 nmol/l. The occurrence of biochemical pregnancy was defined by positive serum hCG, while clinical pregnancy was diagnosed by ultrasound detection of gestational sac with fetal heartbeats. Early pregnancy loss was defined clinically as a first trimester miscarriage. Treatment continued until pregnancy or for up to three induction cycles. Participants who failed to ovulate and remained amenorrheic for ≥6 weeks, withdrawal bleeding was induced with norethisterone treatment.
Statistical methods
Sample size calculation was based on the following equation:
n = required sample size per group
Z∝/2 = 1.96 (the critical value that divides the central 95% of the Z distribution from the 5% in the tail) Zβ = 0.84. The critical value that separates the lower 20% of the Z distribution from the upper 80% and according to previous study by Firouzabadi et al. [8] where p1 = percent of ovulation in the group A who received VD = 56% p2 = percent of ovulation in the group B who didn’t receive VD = 36% q = 1 − p. The calculated sample size was 93 participants in each group.
Data were analyzed using MedCalc© version 18.2.1 (MedCalc© Software bvba, Ostend, Belgium). Continuous numerical variables were presented as mean and SD and intergroup differences were compared using the unpaired t test. Categorical variables were presented as number and percentage, and differences were compared using the Pearson chi-squared test or Fisher’s exact test. Ordinal data were compared using the chi-squared test for trend.
Time to event analysis was done using the Kaplan–Meier (K–M) method. The Mantel–Cox log-rank test was used to compare K–M curves to report the hazard ratio, 95% CI, and p value.
Multivariable binary logistic regression analysis was used to examine the relation between VD supplementation and the occurrence of ovulation or pregnancy as adjusted for the effect of potential confounders. The results were reported as adjusted Odds ratio, 95% CI, and p value. Two-sided p < 0.05 was considered statistically significant.
The number of women needed to be treated with VD to get one positive outcome was presented as the number needed to treat that is the inverse of the absolute risk reduction (ARR), and ARR equals the control event rate minus the experimental event rate.
Results
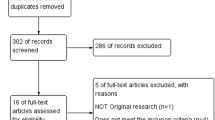
Between January 2018 and September 2018, 208 women were assessed for eligibility. Ten women were ineligible and 12 declined to participate in the study. One hundred and eighty-six women were randomized to the intervention (n = 93) and the placebo (n = 93) groups. None of the women withdrew their consent nor lost to follow-up (Fig. 1).
The demographic characteristics of the participants, the baseline biochemical and hormonal levels and antral follicular count between both groups at the time of randomization did not show a statistically significant difference (Table 1).
Regarding the measured outcomes (registered after 3 months of OI), 86/93 women in the intervention group and 73/93 women in the placebo group had successful ovulation (p = 0.007). Moreover, 57/93 in the VD group versus 46/93 in the placebo group had biochemical pregnancy with p = 0.105, and 47/93 versus 37/93 achieved clinical pregnancy among the interventional and the placebo group respectively (p = 0.141) as shown in Table 2.
The mean diameter of the dominant follicle differed significantly between groups. It was 18.07 ± 1.37 mm in the treatment group, while, the mean diameter of the dominant follicle in the placebo group was 16.79 ± 3.00 mm with p < 0.001. There was no significant difference between groups regarding both preovulatory and mid luteal ET. The ET did not affect the clinical pregnancy rate in both groups with p = 0.98 and 0.213 for preovulatory and mid-luteal ET respectively.
Eight women needed to receive VD supplementation to obtain one successful ovulation, while nine and ten women to obtain one biochemical pregnancy, and one clinical pregnancy, respectively. Moreover, six women needed to receive VD supplementation to avoid one case of early pregnancy loss.
We used K–M curves to report the time from randomization to occurrence of the main outcomes. Median time to ovulation is 1 month in VD group versus 2 months in the placebo group with a statistically significant difference between both K–M curves (log-rank test χ2 = 8.882, df = 1, p = 0.003, hazard ratio = 1.40, 95% CI = 1.03–1.91) (Fig. 2).
K–M curves of the time to biochemical and clinical pregnancy showed that the median time was 3 months in VD group, while the median time could not be determined in the placebo group. There was no statistically significant difference between both K–M curves for biochemical pregnancy (log-rank test χ2 = 2.988, df = 1, p = 0.084, hazard ratio = 1.35, 95% CI = 0.91–1.98), nor for clinical pregnancy (log-rank test χ2 = 1.841, df = 1, p = 0.175, hazard ratio = 1.31, 95% CI = 0.85–2.01) (Supplementary Material 1, 2).
Regarding the cumulative effect over the three induction cycles, ten women should be treated to induce one successful ovulation in the first induction cycle; that increased to 21 women in the third cycle. Moreover, 93 women needed to be treated to have one clinical pregnancy in the first cycle, and only ten women needed to be treated in the third cycle (Supplementary Material 3).
After adjustment for the effect of other variables, (including the age, type and duration of infertility, glucose tolerance and testosterone levels) there was a statistically significant relation between VD supplementation and occurrence of ovulation (odds ratio = 3.884, 95% CI = 1.469–10.268, p = 0.006). There was a statistically significant relation between occurrence of biochemical pregnancy and both VD supplementation (odds ratio = 2.046, 95% CI = 1.062–3.942, p = 0.032), and total testosterone level (odds ratio = 13.469, 95% CI = 4.088–44.380, p < 0.001). There was no statistically significant relation between VD supplementation and occurrence of clinical pregnancy (odds ratio = 1.717, 95% CI = 0.914–3.228, p = 0.093). However, total testosterone was an independent predictor of clinical pregnancy (odds ratio = 8.371, 95% CI = 2.668–26.265, p < 0.001) (Supplementary Material 4).
Multivariable binary logistic regression analysis for the relation between VD supplementation and occurrence of ovulation as adjusted for the effect of hormonal (FSH, LH, E2, TSH, PRL) and metabolic (BMI, glucose tolerance) parameters revealed that VD supplementation was the only significant predictor (Supplementary Material 5).
There was no statistical significant difference between groups regarding side effects of the induction protocol (Table 3).
Discussion
Polycystic ovary syndrome is one of the most common endocrine disorders in women in their reproductive age with variable presentations, as menstrual irregularities, hyperandrogenism, ovulatory dysfunction, insulin resistance (IR), MS, and infertility. Around 67–85% of women with PCOS have serum concentrations of VD levels <20 ng/mL [23].
To the best of our knowledge, the current study is the first double blind RCT to assess the effect of VD supplementation on ovulation rate in overweight anovulatory women with PCOS undergoing OI with clomiphene citrate.
We have found that 92.5% of women received VD had successful ovulation after the induction cycles compared with 78.5% in the placebo group (p = 0.007). The mean diameter of the dominant follicle was 18.07 ± 1.37 mm in the treatment group and was 16.79 ± 3.00 mm with p < 0.001. VD group showed an increase in both the clinical and biochemical pregnancy rates compared to placebo (50.5% and 61.3% versus 39.8% and 49.5% respectively), although this did not reach statistical significance (p = 0.141 and 0.105 respectively).
Eight women needed to receive VD supplementation to obtain one successful ovulation, while nine and ten women to obtain one biochemical pregnancy, and one clinical pregnancy respectively.
After adjustment for the effect of other variables, VD supplementation was the only predictor of the occurrence of ovulation (AOR = 3.884, 95% CI = 1.469–10.268, p = 0.006).Regarding the biochemical pregnancy, both VD supplementation (AOR = 2.046, 95% CI = 1.062–3.942, p = 0.032), and total testosterone level (AOR = 13.469, 95% CI = 4.088–44.380, p < 0.001) were statistically significant.
However, total testosterone was the only independent predictor of clinical pregnancy (AOR = 8.371, 95% CI = 2.668–26.265, p < 0.001) and VD was not statistically significant (AOR = 1.717, 95% CI = 0.914–3.228, p = 0.093).
Ott et al. [16] found that VD deficiency was associated with lower rates of follicle development and pregnancy after CC stimulation. Also Shahrokhi et al. [24] suggested a possible role of VD supplementation in subfertile PCOS women who undergo ovarian stimulation. Also Rashidi et al. found that the number of dominant follicles during the cycles of treatment was higher in the calcium–vitamin D plus metformin group than in either treatment alone (p = 0.03) [25].
Wehr et al. [22] found that VD treatment for 12 weeks in women with PCOS improved menstrual irregularity in 30.4% of women and 4 out of 16 women seeking pregnancy at baseline conceived (25%).
Pal et al. [26] in their secondary analysis of the Pregnancy in PCOS I (PPCOS I) multicenter RCT found that the ovulation varied directly with VD levels in sampled cohort (68%, 77%, and 78% in those with VD deficiency, insufficiency, and normal status, respectively; p = 0.050). With significant better ovulation rate with VD levels ≥20 ng/mL (p = 0.006).
Moreover, VD level was significantly higher in women achieved live birth (LB) compared with those failing to attain LB with each 1 ng/mL increase in serum VD increased the likelihood of LB by 2% (OR, 1.02; 95% CI, 1.00, 1.04; p = 0.046).
In the current study the ET did not affect clinical pregnancy rate in both groups with p = 0.98 and 0.213 for preovulatory and mid luteal ET respectively. In the contrast of Ozkan et al. who reported the lack of correlation between VD levels and ovarian response parameters, suggesting that the endometrium and endometrial receptivity may be the site of VD action enhancing the reproductive success [27]. Furthermore, this concept of the endometrial effect of VD was supported by Rudick et al. [9] who found that VD played an important role in IVF pregnancies, possibly via localized effects in the endometrium.
Excess androgens is a main factor encouraging the development of the multiple small follicles characteristic of the polycystic ovaries that produces anti-mullerian hormone in significantly increased concentrations that counteract the actions of FSH causing anovulation [28]. Increased subfertility, ectopic pregnancy, and early pregnancy loss rates in PCOS were linked to the altered endometrial environment and subsequent reduction in implantation due to the hyperinsulinemic environment and concurrent hyperandrogenism [29]. Elevated testosterone in women with PCOS is associated with decreased rates of fertilization and embryonic development. In addition, elevated testosterone concentrations are associated with higher miscarriage rates, suggesting that androgens may have a detrimental effect on folliculogenesis and endometrial function [30].
Conclusion
In subfertile women with PCOS, VD supplementation with ovulation induction significantly improved the ovulation rate with no significant effect on clinical or biochemical pregnancy rates.
Strengths and limitations
To our knowledge, this study is the first randomized placebo-controlled trial on VD supplementation on ovulation and clinical pregnancy rates in women with PCOS undergoing induction of ovulation. All previous studies targeted the effect of VD deficiency on menstrual regularity, IR, and infertility or the therapeutic effect of VD on weight loss, follicle maturation, menstrual regularity, and improvement of hyperandrogenism in infertile women with PCOS.
However, our study had several limitations. Importantly, it was performed at a single center on a small sample size without measuring VD level. however VD deficiency is considered the most prevalent nutritional deficiency worldwide (reaching 72% of women in childbearing periods in Egypt and 85% of women with PCOS). VD supplementation is accessible, widely available, and safe as in healthy individuals. Exogenous VD intoxication is usually caused by prolonged use (months) of VD mega doses. Future multicenter study on a bigger sample size is required. Moreover, long-term follow up is strongly recommended to assess whether VD supplementation for longer induction cycles can get better clinical pregnancy rate and LB rates.
Change history
03 December 2021
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s12020-021-02946-0
References
R.L. Thomson, S. Spedding, J.D. Buckley, Vitamin D in the etiology and management of polycystic ovary syndrome. Clin. Endocrinol. 77, 343–350 (2012). https://doi.org/10.1111/j.1365-2265.2012.04434.x
H. Teede, A. Deeks, L. Moran, Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts health across the lifespan. BMC Med. 8, 41–48 (2010). https://doi.org/10.1186/1741-7015-8-41
A. Hossein-nezhad, M.F. Holick, Optimize dietary intake of vitamin D: an epigenetic perspective. Curr. Opin. Clin. Nutr. Metab. Care 15(6), 567–579 (2012). https://doi.org/10.1097/MCO.0b013e3283594978
M.J. Bolland, A. Grey, A. Avenell, Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 6(11), 847–858 (2018)
K.Y.Z. Forrest, W.L.Stuhldreher, Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 31(1), 48–54 (2011). https://doi.org/10.1016/j.nutres.2010.12.001
C. He, Z. Lin, S.W. Robb, A.E. Ezeamama, Serum vitamin D levels and polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients 7(6), 4555–77 (2015). https://doi.org/10.3390/nu7064555
H. Selimoglu, C. Duran, S. Kiyici, C. Ersoy, M. Guclu, G. Ozkaya, E. Tuncel, E. Erturk, S. Imamoglu, The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J. Endocrinol. Investig. 33(4), 234–238 (2010). https://doi.org/10.3275/6560
R. Firouzabadi, A. Aflatoonian, S. Modarresi, L. Sekhavat, S. MohammadTaheri, Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement. Ther. Clin. Pract. 18(2), 85–88 (2012). https://doi.org/10.1016/j.ctcp.2012.01.005
B. Rudick, S. Ingles, K. Chung, F. Stanczyk, R. Paulson, K. Bendikson, Characterizing the influence of vitamin D levels on IVF outcomes. Hum. Reprod. 27(11), 3321–3327 (2012). https://doi.org/10.1093/humrep/des280
R. Yildizhan, M. Kurdoglu, E. Adali, A. Kolusari, B. Yildizhan et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 280, 559–563 (2009). https://doi.org/10.1007/s00404-009-0958-7
R.M. Botros, I.M. Sabry, R.S. Abdelbaky, Y.M. Eid, M.S. Nasr, L.M. Hendawy, Vitamin D deficiency among healthy Egyptian females. Endocrinol. Nutr. 62(7), 314–321 (2015). https://doi.org/10.1016/j.endonu.2015.03.010
M. Irani, Z. Merhi, Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil. Steril. 102, 460–468 (2014). https://doi.org/10.1016/j.fertnstert.2014.04.046
S. Mazloomi, F. Sharifi, R. Hajihosseini, S. Kalantari, S. Mazloomzadeh, Association between hypoadiponectinemia and low serum concentrations of calcium and vitamin D in women with polycystic ovary syndrome. ISRN Endocrinol. 949427 (2012). https://doi.org/10.5402/2012/949427
L. Pal, A. Berry, L. Coraluzzi, E. Kustan, C. Danton, J. Shaw et al. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol. Endocrinol. 28, 965–968 (2012). https://doi.org/10.3109/09513590.2012.696753
A.Z.E. Alshaymaa, R. Rasha Ali, G. Md Zakaria, M.d. Sahar, M. Abdel, A closer insight into the role of vitamin D in polycystic ovary syndrome (Pcos). Glob. J. Pharm. Sci. 6(4), 555692 (2018). https://doi.org/10.19080/GJPPS.2018.06.555692
J. Ott, L. Wattar, C. Kurz, R. Seemann, J.C. Huber, K. Mayerhofer, E. Vytiska-Binstorfer, Parameters for calcium metabolism in women with polycystic ovary syndrome who undergo clomiphene citrate stimulation: a prospective cohort study. Eur. J. Endocrinol. 166, 897–902 (2012). https://doi.org/10.1530/EJE-11-1070
S.F. Butts, D.B. Seifer, N. Koelper, S. Senapati, M.D. Sammel, A.N. Hoofnagle et al., Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J. Clin. Endocrinol. Metab. (2018). https://doi.org/10.1210/jc.2018-00750
J. Luk, S. Torrealday, G.N. Perry, L. Pal, Relevance of vitamin D in reproduction. Hum. Reprod. 27(10), 3015–3027 (2012). https://doi.org/10.1093/humrep/des248
M.F. Holick, Vitamin D deficiency. N. Engl. J. Med 357(3), 266–281 (2007). https://doi.org/10.1056/NEJMra070553
D. Shah, P. Gupta, Vitamin D deficiency: is the pandemic for real? Indian J. Community Med 40(4), 215–217 (2015). https://doi.org/10.4103/0970-0218.164378
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81, 19–25 (2004). https://doi.org/10.1016/j.fertnstert.2003.10.004
E. Wehr, T.R. Pieber, B. Obermayer-Pietsch, Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in polycystic ovary syndrome women: a pilot study. J. Endocrinol. Investig. 34(10), 757–763 (2011). https://doi.org/10.3275/7748
A. Nandi, N. Sinha, E. Ong, H. Sonmez, L. Poretsky, Is there a role for vitamin D in human reproduction? Horm. Mol. Biol. Clin. Investig. 25(1), 15–28 (2016). https://doi.org/10.1515/hmbci-2015-0051
S.Z. Shahrokhi, F. Ghaffari, F. Kazerouni, Role of vitamin D in female reproduction. Clin. Chim. Acta 455, 33–38 (2016). https://doi.org/10.1016/j.cca.2015.12.040
B. Rashidi, F. Haghollahi, M. Shariat, F. Zayerii, The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J. Obstet. Gynecol. 48(2), 142–147 (2009). https://doi.org/10.1016/S1028-4559(09)60275-8
L. Pal, H. Zhang, J. Williams et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J. Clin. Endocrinol. Metab. 101(8), 3027–3035 (2016). https://doi.org/10.1210/jc.2015-4352
S. Ozkan, S. Jindal, K. Greenseid, J. Shu, G. Zeitlian, C. Hickmon et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil. Steril. 94, 1314–1319 (2010). https://doi.org/10.1016/j.fertnstert.2009.05.019
R. Homburg, Androgen circle of polycystic ovary syndrome. Hum. Reprod. 24(7), 1548–1555 (2009). https://doi.org/10.1093/humrep/dep049
R. McDonnell, R.J. Hart, Pregnancy-related outcomes for women with polycystic ovary syndrome. Women’s Health 13(3), 89–97 (2017). https://doi.org/10.1177/1745505717731971
J. Qiao, H.L. Feng, Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 17(1), 17–33 (2011). https://doi.org/10.1093/humupd/dmq032
Acknowledgements
We are grateful to all staff that helped with the trial including infertility clinic nurses, and sonographers.
Author information
Authors and Affiliations
Contributions
R.R. contributed in literature search, study design, data analysis, interpretation, and drafted the article. S.H. contributed in literature search, study design, data analysis, interpretation, and revised the article; E.A. contributed in literature search, study design, data analysis, interpretation, and revised the article; and S.Y. was responsible for data collection.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This trial was approved by the Research Review Board of the Obstetrics and Gynecology Department, Faculty of medicine, Ain Shams University. The study methodology was registered on clinical trials.gov: NCT03396380. It was conducted and reported in accordance with CONSORT guidelines for reporting randomized trials.
Informed consent
Any woman eligible to participate was informed about the trial by one of the researchers and those approved to participate gave informed written consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasheedy, R., Sammour, H., Elkholy, A. et al. The efficacy of vitamin D combined with clomiphene citrate in ovulation induction in overweight women with polycystic ovary syndrome: a double blind, randomized clinical trial. Endocrine 69, 393–401 (2020). https://doi.org/10.1007/s12020-020-02315-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02315-3