Abstract
Objective
To analyze the prognostic influence of patient characteristics, diagnostic markers or therapeutic procedures in women diagnosed with early ovarian cancer based on relapse and survival in long term follow-up.
Materials and methods
All women diagnosed and treated for early ovarian cancer at our institution between 1992 and 2006 were included in this retrospective study. Patient characteristics, clinical data including operative procedure, serum markers, stage and histology at first diagnosis as well as follow-up data were analyzed with regard to survival times and relapse rates.
Results
Altogether, 116 patients were included. Mean follow-up time was 7.0 ± 3.3 years (range 2–14 years). Histology revealed a serous tumor in 64.7% (75/116), mucinous in 19.0% (22/116) and endometiroid tumors in 7.8% (9/116) of all cases. TNM classification was pT1a in 49.1% (57/116), pT1b in 6% (7/116), pT1c in 32.8% (38/116) and pT2a in 12.1% (14/116). Lymph node involvement (N1) was found in 3.4% of all patients. 17 deaths and 17 relapses (each 14.7%) were documented during follow-up time with a mean time to recurrence of 3.3 ± 2.1 years (range 1–7 years). The general 1-, 2-, 5- and 10-year survival rates were 99, 95.7 and 88.9 and 81.0%, respectively. Patients with tumor stage pT1a and pT1b had a significantly better survival (P = 0.0003) and significantly lower risk of recurrence (P = 0.0138) compared to higher tumor stages. Moreover, patients who experienced recurrent disease or presented with ascites at primary diagnosis had a significantly worse overall survival (recurrence: hazard ratio 0.17, 95% confidence interval 0.0155–0.2182, P = 0.0001; ascites: HR 2.84, CI 1.1919–10.1131, P = 0.0225). The risk for recurrent disease was significantly elevated for patients with low grade (G3) tumors (P = 0.0330). Interestingly, there was neither a worse survival rate nor a higher relapse rate for patients with primary laparoscopic surgical access.
Conclusion
Patients with early ovarian cancer stage pT1c and pT2a or low grade tumor have to be monitored closely in oncologic follow-up as they bare a significant risk for disease recurrence. Ascites at primary diagnosis, pT1c or pT2a tumor stage or recurrent disease are associated with a poor survival even in early ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the leading cause of death among gynecologic malignancies. Due to the lack of diagnostic tools for early detection of ovarian cancer, the vast majority is detected at progressed stage of disease. Only about 25–30% of all patients are diagnosed as early ovarian cancer (EOC) [8]. Though macroscopically limited to the ovaries, there is a high incidence of microscopic tumor spread, which has been described to range around 30% in earlier studies [23, 31]. If those patients are not staged adequately, there is a high risk to underestimate the stage of the disease and the necessity for adjuvant therapy. Therefore, a sufficient operative staging is mandatory in all patients diagnosed with ovarian cancer [24]. Although longitudinal laparotomy is still the standard surgical access, laparoscopy is frequently preferred as minimally invasive approach to evaluate unclear adnexal masses because it is well accepted by patients, only requires a short hospitalization and entails less postoperative pain [13].
The aim of this study is to analyze the prognostic influence of patient characteristics, diagnostic markers or therapeutic procedures in women diagnosed with early ovarian cancer based on relapse and survival in long term follow-up.
Materials and methods
All women diagnosed and treated for early ovarian cancer (EOC) (pT1a–T2a) at our institution between 1992 and 2006 were included in this retrospective study. Patient characteristics, clinical data including operative procedure, evaluation of serum CA-125 levels (automated enzyme immunoassay, Elecsys, Roche Diagnostics, Penzberg, Germany), stage and histology at first diagnosis as well as follow-up data were analyzed for survival times and relapse rates. Clinical data, demographic, diagnostic and treatment information were primarily collected from the patients’ charts. Further follow-up was carried out by outpatient visits and tumor registry data base information.
Patients were seen three-monthly after initial diagnosis for a 2-year-period, thereafter at a 6-month interval for another three years and afterwards once a year to evaluate tumor markers, sonographic and clinical signs of relapse. The following parameters were registered for each patient: age at primary diagnosis, menopausal stage, hormone therapy, tumor marker, ascites, surgical procedure performed, adjuvant therapy, tumor type and stage. Tumor typing and staging were performed by the department of pathology according to the criteria of the International Federation of Gynaecologists and Obstetricians (FIGO) and the International Union against Cancer (IUCC). In follow-up, occurrence of relapse, time to relapse, death and survival time were registered. The main outcomes assessed were disease recurrence and patients’ survival.
Statistical analysis was performed using Medcalc (8.1). All values are given as mean and standard deviation. To test differences between continuous variables for statistical significance, the Mann–Whitney test for unpaired variables was applied. For categorical data, the chi square test was used. For the comparison of survival times, Kaplan–Meier curves were drawn for different patient groups. The chi-square statistic of the logrank test was calculated to test differences between survival curves for significance. P values less than 0.05 were considered as statistically significant.
Results
Altogether, 116 patients could be identified who had been operated und diagnosed with early ovarian cancer at our institution between 1992 and 2006.
Mean follow-up time was 7.0 ± 3.3 years (range 2–14 years). Mean age at primary diagnosis was 55 years (minimum 14 years, maximum 88 years). 2.6% of the patients were premenopausal, 21.9% perimenopausal and 75.5% postmenopausal. At primary diagnosis, serum tumor marker CA 125 was elevated above the cut off of 35 U/ml in 70% of all patients.
Histology revealed a serous tumor in 64.7% (75/116), mucinous in 19.0% (22/116) and endometiroid in 7.8% (9/116) of all cases. All other histological subtypes ranged between 0.9 and 1.7%. TNM classification was pT1a in 49.1% (57/116), pT1b in 6% (7/116), pT1c in 32.8% (38/116) and pT2a 12.1% (14/116). Lymph node involvement (N1) was found in 3.4% of all patients.
The tumor grading was G1 in 33.6%, G2 in 44.8% G3 in 19.0%. Further analysis of tumor grading according to the histological subtype showed a G1 differentiation in 30.7% of all serous tumors, G2 in 44.0%, G3 in 22.7%. In comparison to these numbers, the second most common tumor type, mucinous ovarian cancer, showed a better grading: G1 in 59.1%, G2 in 31.8%, G3 in 4.5%. Ascites was discovered in 28.4% (33/116) of all patients at primary diagnosis (Table 3).
The surgical access was longitudional laparotomy in 81.9% (n = 95 patients), transverse laparotomy in 0.9% (n = 1 patient) and laparoscopy in 17.2% (n = 20 patients) at initial surgery. An intraoperative conversion from laparoscopic to laparotomic approach did not occur. A separate second operation was necessary in 19 cases, which accounts for as much as 95% of all patients with invasive ovarian carcinoma and primary laparoscopic operative access (19/20). In case of a secondary surgical access, it was laparoscopic in 7 cases (7/19) and laparotomic in 12 (12/19). No port site metastases were diagnosed during follow-up.
Adjuvant chemotherapy was administered in about half of all patients, i.e. 50.9%. A combination of carboplatin and paclitaxel was chosen in 44.8% of the patients treated by chemotherapy, while a combination of carboplatin and cyclophosphamide was applied in 34.5%. Other combinations were cisplatin and paclitaxel or carboplatin, paclitaxel and cyclophosphamide, each in 3.5% of patients. Altogether 86.2% of all patients receiving chemotherapy were treated with a combined chemotherapeutic strategy. Carboplatin monotherapy was chosen in 10.3%. One patient was treated with cyclophosphamide alone and one with cisplatin. 75.9% of all patients had six cycles of chemotherapy, 17.1% four cycles and the other 7.0% varied from two to eight or ten cycles altogether.
A total of 17 relapses (14.7%, 17/116) were documented during follow-up time with a mean time to recurrence of 3.3 ± 2.1 years (range 1–7 years). Of all patients, 17 died during follow-up time. The general 1-, 2-, 5- and 10-year survival rates were 99, 95.7 and 88.9 and 81.0%, respectively.
Patient survival
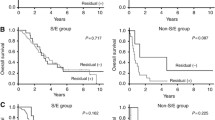
Of the 17 patients who died, nine had been operated for recurrent disease during follow-up time. Kaplan–Meier analysis showed a significant difference in terms of survival for patients who experienced disease recurrence or not. For patients without disease recurrence, 1-, 2-, 5- and 10-year survival rates were 100, 99, 93.4 and 88.5 compared to 94.1, 76.5, 64.7 and 49.3% in those with relapse (log rank test: HR 0.17, CI 0.0155–0.2182, P = 0.0001; cf. Fig. 1).
Tumor stage was significantly relevant for overall survival: pT1c and pT2a ovarian cancer had the worst 1-, 2- and 5-year survival rates, whereas pT1b tumors showed the best survival data with a 5-year survival of 100% (P = 0.0003; cf. Table 1). Mean survival was 6.5 ± 2.4 years for pT1a, 12.0 ± 0 years for pT1b, 2.0 ± 0.8 years for pT1c and 4.0 ± 1.4 years for pT2a. Patients with a low grade tumor (G3) had a significantly higher tumor stage at primary diagnosis (P < 0.01). Besides, a significant correlation could be found for elevated CA 125 (>35 U/ml) at primary diagnosis and higher tumor stage (P = 0.001).
Grading, histological subtype and CA 125 elevation at primary diagnosis did not show a significant difference in terms of overall survival (P > 0.05 in the log rank test of Kaplan–Meier analysis). Interestingly, the primary surgical access, either by laparoscopy or laparotomy, had no influence on overall survival rates with a 5-year survival rate of 88.7% in patients with laparoscopy versus 88.9% in those with laparotomy (HR 0.80, CI 0.2008–3.2224, P = 0.760; cf. Fig. 2, Table 4).
Another significant correlation was observed between the presence or absence of ascites at primary diagnosis and survival (HR 2.84, CI 1.1919–10.1131, P = 0.0225; cf. Figs. 3).
Relapse
17 of 116 patients (14.7%) were diagnosed with recurrent disease during follow-up. Relapse free 1-, 2-, 5- and 10-year interval for all patients were 96.6, 92.2, 87.9 and 81.4%, respectively. Patients with pT1a and pT1b tumor stage showed a significantly better relapse free interval than pT1c and pT2a tumors patients, with a 1-, 2- and 5-year relapse free interval of 98.2, 94.7 and 94.7% for pT1a, 5-year relapse free interval of 100% for pT1b, rates of 94.7, 89.5 and 77.8% for pT1c tumors and 92.9, 85.7 and 77.9 for pT2a tumors (P = 0.0138; cf. Table 2).
Tumor marker elevation at primary diagnosis, the presence of ascites or histological subtype did not show significant differences in terms of relapse rates (P > 0.05 for log-rank tests of Kaplan–Meier analyses of all parameters). Again the choice of primary surgical access had no influence on patients’ recurrence rates: relapse free 5-year interval was 95.0% with laparoscopy versus 86.4% with laparotomy (HR 0.2425, CI 0.1236–1.6966, P = 0.324; Table 4). The risk of recurrent disease was significantly elevated for patients with low grade (G3) tumors (P = 0.0330) (Fig. 4).
Discussion
Persistent ovarian masses of unclear dignity found at subsequent vaginal sonographies result in operative exploration in the vast majority of cases. Although most lesions turn out to be benign, some are diagnosed as invasive ovarian cancers [5]. Since laparoscopic minimally invasive surgery gains importance for its faster postoperative recovery and shorter stay in hospital [13], women with unclear ovarian mass often wish to be operated laparoscopically, baring the risk to require complementary surgery, mostly by laparotomy, afterwards.
Park et al. compared the operative procedures for early ovarian cancer. In a short follow-up time they also found no difference in terms of relapse or survival times between both patients groups [19]. Our data are also in concordance with Lecuru et al. [14] who did not find a harmful influence of laparoscopy as first initial access on outcomes of patients with stage I ovarian cancer, although they found insufficient radicality and inaccurate staging with initial laparoscopy. Ghezzi et al. [6] found laparoscopic comprehensive surgical staging of EOC to be a safe and adequate treatment option comparable to the standard surgical staging performed via laparotomy.
In our patient group, patients treated by laparoscopy had a significantly higher risk to require a second operation. An explanation for this observation is the patients’ wish for a minimally invasive surgery and an elucidation of the histologic result prior to complete ovarian cancer surgery. None of the patients treated by laparoscopy showed port site metastasis in our patient group, which is a commonly described concern related to laparoscopy. In literature this phenomenon has been described in a few cases [1, 7, 9, 10, 16, 17, 27–29], mostly associated with tumor spillage. Others have criticized the delay of secondary surgery after laparoscopy to be relevant for outcome [11, 15]. But this concern should be addressed to both operative access, laparotomy and laparoscopy, if a stepwise surgical approach is chosen.
A study of the Regional Cancer Registry of the central region in the Netherlands has underlined the importance of proper surgical staging. They compared patients who had been treated according to the guidelines for ovarian cancer surgery to those who had not been operated accordingly, and showed a significant difference of 29.1% between the completely and incompletely staged group in the 5-year survival in Kaplan–Meier analysis [21]. Moreover, they postulated that there are patients with higher tumor stages among the patients without proper surgery who remain under-diagnosed at surgery [21]. This concern has already been raised by others who found EOC patients at risk to miss metastases at incomplete surgery, which consecutively has significant implications on treatment and survival [12, 20]. Since there are no data of randomized prospective trials available, the primary operative procedure for invasive ovarian cancer should include longitudinal laparotomy, bilateral salpingo-oophorectomy, hysterectomy, omentectomy, pelvic and paraaortal lymphadenectomy, multiple peritoneal biopsies and peritoneal washing [3, 24].
Our data show tumor stage to be an independent prognostic factor for relapse and survival. This finding is supported by other studies on EOC [2, 18, 30]. Five year survival rates are as high as 98.1 and 100% in stage pT1a and pT1b disease, but only 86.7 and 50.8% for pT1c and pT2a tumors. These 5-year survival rates go along with the data in literature showing a 5-year survival rate in stage I cancers of 84% compared with 76% in those with stage II disease [4]. The high prognostic relevance of TNM-stage observed in this study underlines that all ovarian cancer have to be staged properly, especially to guarantee adequate adjuvant treatment.
To detect EOC patients with poor survival or high risk of recurrence, independent prognostic tools have been analyzed. In this study, we identified ascites at primary diagnosis and a low tumor grading as risk factors. Vergote et al. [27] analyzed the data of 1,545 women with stage I disease and found a low tumor grade to be associated with a shorter disease free survival, which is in agreement with our observation. Other studies support this finding [12, 22, 25, 26], like John et al. who also described tumor grade to be an independent prognostic factor for progression-free and disease-free survival [4].
Strengths of this study are the long follow-up of 14 years, the persistent high standard of operative staging by gynecologic oncologists at a specialized academic institution and consistent pathologic histology review by expert gynecologic oncology pathologists. A limitation is obviously the retrospective, non-randomized study design and changes in adjuvant chemotherapy regimens over the long observation period.
Conclusion
In conclusion, patients with tumor stage pT1c and pT2a or low grade tumor have to be monitored closely in oncologic follow-up as they bare a significant risk for disease recurrence. Ascites at primary diagnosis and pT1c or pT2a tumor stage or disease recurrence are associated with a poor overall survival. Since TNM-stage is of such prognostic relevance, even patients with early ovarian cancer have to be staged properly to ensure adequate adjuvant treatment. Laparoscopy as initial surgical access has no negative influence on recurrence or survival and can be regarded as adequate for initial diagnostic surgery.
References
Abu-Rustum NR, Rhee EH, Chi DS et al (2004) Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease. Obstet Gynecol 103:480–487
Bertelsen K, Holund B, Andersen JE et al (1993) Prognostic factors and adjuvant treatment in early epithelial ovarian cancer. Int J Gynecol Cancer 3:211–218. doi:10.1046/j.1525-1438.1993.03040211.x
Canis M, Jardon K, Niro J et al (2007) Endoscopic management of gynecological malignancies: an update. Bull Acad Natl Med 191:1357–1365 (discussion 1365–1366)
Chan JK, Tian C, Monk BJ et al (2008) Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer 112:2202–2210. doi:10.1002/cncr.23390
Fishman DA, Cohen L, Blank SV et al (2005) The role of ultrasound evaluation in the detection of early-stage epithelial ovarian cancer. Am J Obstet Gynecol 192:1214–1221. doi:10.1016/j.ajog.2005.01.041 discussion 1221–1212
Ghezzi F, Cromi A, Uccella S et al (2007) Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol Oncol 105:409–413. doi:10.1016/j.ygyno.2006.12.025
Gleeson NC, Nicosia SV, Mark JE et al (1993) Abdominal wall metastases from ovarian cancer after laparoscopy. Am J Obstet Gynecol 169:522–523
Heintz AP, Odicino F, Maisonneuve P et al (2006) Carcinoma of the ovary. FIGO 6th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 95(suppl 1):S161–S192. doi:10.1016/S0020-7292(06)60033-7
Hopkins Mp, Von Gruenigen V, Gaich S (2000) Laparoscopic port site implantation with ovarian cancer. Am J Obstet Gynecol 182:735–736. doi:10.1067/mob.2000.103251
Huang KG, Wang CJ, Chang TC et al (2003) Management of port-site metastasis after laparoscopic surgery for ovarian cancer. Am J Obstet Gynecol 189:16–21. doi:10.1067/mob.2003.330
Kindermann G, Maassen V, Kuhn W (1995) Laparoscopic preliminary surgery of ovarian malignancies. Experiences from 127 German gynecologic clinics. Geburtshilfe Frauenheilkd 55:687–694
Le T, Adolph A, Krepart GV et al (2002) The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecol Oncol 85:351–355. doi:10.1006/gyno.2002.6636
Leblanc E, Sonoda Y, Narducci F et al (2006) Laparoscopic staging of early ovarian carcinoma. Curr Opin Obstet Gynecol 18:407–412. doi:10.1097/01.gco.0000233935.51801.48
Lecuru F, Desfeux P, Camatte S et al (2006) Impact of initial surgical access on staging and survival of patients with stage I ovarian cancer. Int J Gynecol Cancer 16:87–94. doi:10.1111/j.1525-1438.2006.00303.x
Lehner R, Szabo S, Goharkhy N et al (2001) Prognostic influence of delays between exploratory and definitive laparotomy in the treatment of malignant ovarian tumors. Arch Gynecol Obstet 265:36–39. doi:10.1007/s004040000125
Morice P, Viala J, Pautier P et al (2000) Port-site metastasis after laparoscopic surgery for gynecologic cancer. A report of six cases. J Reprod Med 45:837–840
Nagarsheth NP, Rahaman J, Cohen CJ et al (2004) The incidence of port-site metastases in gynecologic cancers. Jsls 8:133–139
Nagele F, Petru E, Medl M et al (1995) Preoperative CA 125: an independent prognostic factor in patients with stage I epithelial ovarian cancer. Obstet Gynecol 86:259–264. doi:10.1016/0029-7844(95)00126-C
Park JY, Kim Dy, Suh DS et al (2008) Comparison of laparoscopy and laparotomy in surgical staging of early-stage ovarian and fallopian tubal cancer. Ann Surg Oncol 15:2012–2019
Pomel C, Jeyarajah A, Oram D et al (2007) Cytoreductive surgery in ovarian cancer. Cancer Imaging 7:210–215. doi:10.1102/1470-7330.2007.0030
Sijmons EA , van Lankveld VL, Witteveen Po et al (2007) Compliance to clinical guidelines for early-stage epithelial ovarian cancer in relation to patient outcome. Eur J Obstet Gynecol Reprod Biol 131:203–208. doi:10.1016/j.ejogrb.2006.03.014
Skirnisdottir I, Sorbe B (2007) Survival and prognostic factors in early-stage epithelial ovarian carcinoma treated with taxane-based adjuvant chemotherapy. Int J Gynecol Cancer 17:1231–1237. doi:10.1111/j.1525-1438.2007.00928.x
Soper JT, Johnson P, Johnson V et al (1992) Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol 80:949–953
Colomer TA, Jimenez AM, Bover Barcelo MI (2008) Laparoscopic treatment and staging of early ovarian cancer. J Minim Invasive Gynecol 15:414–419
Trimbos JB, Vergote I, Bolis G et al (2003) Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European organisation for research and treatment of cancer-adjuvant chemotherapy in ovarian neoplasm trial. J Natl Cancer Inst 95:113–125
Trope C, Kaern J, Hogberg T et al (2000) Randomized study on adjuvant chemotherapy in stage I high-risk ovarian cancer with evaluation of DNA-ploidy as prognostic instrument. Ann Oncol 11:281–288. doi:10.1023/A:1008399414923
Vergote I, De Brabanter J, Fyles A et al (2001) Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 357:176–182. doi:10.1016/S0140-6736(00)03590-X
Vergote I, Marquette S, Amant F et al (2005) Port-site metastases after open laparoscopy: a study in 173 patients with advanced ovarian carcinoma. Int J Gynecol Cancer 15:776–779. doi:10.1111/j.1525-1438.2005.00135.x
Weiss EG, Wexner SD (1996) Laparoscopic port site recurrences in oncologic surgery—a review. Ann Acad Med Singapore 25:694–698
Young RC, Brady MF, Nieberg RK et al (2003) Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin—a gynecologic oncology group study. J Clin Oncol 21:4350–4355. doi:10.1200/JCO.2003.02.154
Young RC, Decker DG, Wharton JT et al (1983) Staging laparotomy in early ovarian cancer. JAMA 250:3072–3076. doi:10.1001/jama.250.22.3072
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lenhard, S.M., Bufe, A., Kümper, C. et al. Relapse and survival in early-stage ovarian cancer. Arch Gynecol Obstet 280, 71–77 (2009). https://doi.org/10.1007/s00404-008-0877-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-008-0877-z