Abstract
Background
The aim of the present study was to investigate the incidence and hallmarks of long-term survivors of recurrent ovarian carcinoma (LTSROC) in a large-scale retrospective cohort of patients from a multicenter study group.
Methods
We performed a regional multicenter retrospective study between January 1986 and September 2021 using clinical data collected under the central pathological review system. Patients who underwent surgery for primary OC at diagnosis and developed recurrent tumors after the initial treatment were included. We defined LTSROC as patients who survived for 5 years or longer after initial tumor recurrence and examined factors affecting the long-term survival of ROC and outcomes of LTSROC.
Results
We collected information on patients with malignant ovarian tumors and finally 657 of them that developed ROC were included in the study population. Sixty-eight (10.4%) patients were LTSROC while 399 (60.7%) were short-term survivors of recurrent ovarian carcinoma. In a multivariate logistic regression analysis, negative ascites cytology [odds ratio (OR) 1.865; 95% CI 1.026–3.393; p = 0.041] and a recurrence-free interval (RFI) of 1 year or longer (OR 2.896; 95% CI 1.546–5.425; p < 0.001) were identified as independent factors associated with LTSROC. Approximately 80% of LTSROC presented with solitary recurrent tumors. Furthermore, more than 50% of LTSROC underwent tumor debulking surgery for the first recurrent tumor with or without chemotherapy.
Conclusion
RFI of 1 year or longer and negative ascites cytology in the initial surgery were identified as independent predictive factors for LTSROC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian carcinoma (OC) is one of the most aggressive gynecologic malignancies worldwide, with more than 214,100 newly diagnosed cases and 137,700 reported deaths per year in U.S [1]. OC is considered to originate from ovarian surface epithelial or neighboring fallopian tubal cells, and is also known as a so-called “silent killer” because most women with ovarian carcinoma are generally asymptomatic until progressing to an advanced stage [2]. Even though complete clinical remission may be achieved in approximately 80% of these patients by cyto-reductive surgery, followed by systemic front-line chemotherapy, the majority of clinical complete responders develop recurrent disease [3].
Most women who develop recurrent OC (ROC) will be offered further chemotherapy with the likelihood of a survival benefit related in part to the initial response to chemotherapy and the duration of the response [4]. The aims of treatment for ROC are the prolongation of survival times, the control of tumor progression and disease-related symptoms, and the maintenance of quality of life [5]. The survival outcome of ROC is reported to be extremely poor with most patients dying from the disease [6]. On the other hand, a small group of patients achieve long-term post-recurrence survival in the presence or absence of disease. Although previous studies reviewed long-term survivors with ROC (LTSROC) [7, 8], limited information is currently available on the characteristics of this group due to its rarity and the difficulties associated with long-term follow-ups. Therefore, the identification of factors contributing to LTSROC as well as the recognition of its clinical features will be important for gynecologic oncologists to increase the possibility of long-term survival.
To obtain further information on the incidence and hallmarks of LTSROC, we herein analyzed a large-scale retrospective cohort of patients with ROC from a multicenter study group. We also aimed to clarify the factors affecting the long-term survival of ROC and evaluate the outcomes of LTSROC to identify the clinical features and common salvage treatments that influenced post-recurrence survival.
Materials and methods
Study participants
The present study was a regional retrospective cohort study performed between January 1986 and September 2021 with the Tokai Ovarian Tumor Study Group, consisting of Nagoya University and its affiliated hospitals. The Ethics Committee of Nagoya University approved the present centralized study in accordance with the principles of the Declaration of Helsinki. The histology of OC was assessed according to the criteria of the World Health Organization classification [9], under the central pathological review system of the Tokai Ovarian Tumor Study Group. Additionally, the clinical stage of patients was assigned with the staging system of the International Federation of Gynecology and Obstetrics [10].
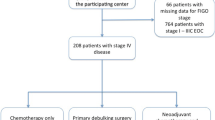
We initially included patients who underwent surgery for primary OC at diagnosis and developed recurrent tumors after the initial treatment. Patients without sufficient clinical data or lost to follow-up immediately after tumor recurrence were excluded. We defined LTSROC as patients who survived for 5 years of longer after initial tumor recurrence because the term was one of the usual follow-up periods for cancer patients. In contrast, short-term survivors with ROC (STSROC) were defined as patients who survived for less than 5 years after the first recurrence. After selecting LTSROC, we then excluded patients without sufficient clinical data on the treatment for recurrent tumors and examined the characteristics of patients with no evidence of disease (NED) and alive with disease (AWD) (Fig. 1).
Surgery, chemotherapy, and follow-up
Primary staging laparotomy was performed on all patients to assess the abdominal contents. It principally consisted of hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and retroperitoneal lymphadenectomy or sampling. A peritoneal evaluation with cytology, palpation, and/or biopsy was routinely performed in the initial debulking surgery. Patients with advanced-stage OC underwent maximal cyto-reduction. Secondary debulking surgery for ROC was performed for completely resectable tumors at each surgeon’s discretion. In the present study, we defined a complete operation as staging laparotomy with hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and pelvic/para-aortic lymphadenectomy. A standard operation comprised staging laparotomy with hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and/or incomplete retroperitoneal lymphadenectomy. Probe laparotomy was a staging operation without any organ resection including the uterus, ovary, or omentum. Regarding chemotherapy, we basically used the same selection criteria for first-line regimens as the Tokai Ovarian Tumor Study Group. Details on chemotherapy during each period were previously described [11]. Monotherapy avoiding cross-resistance to a previous regimen was selected for ROC after a disease-free interval of less than 6 months. In contrast, combination therapy with a platinum agent was mainly performed for ROC after a disease-free interval of longer than 6 months.
All patients were followed up at an outpatient office at each institution with ultrasonography, an evaluation of CA-125 levels, and a periodic computed tomographic scan. Positron emission tomography was performed according to the discretion of the attending physician. In the present study, the recurrence-free interval (RFI) was defined as the time interval between the date of initial debulking surgery and the diagnosis of recurrence. On the other hand, post-recurrence survival was defined as the time interval between the date of recurrence and the last date of the follow-up visit or death due to the disease. We selected 1 year as the cut-off value for RFI because of a lack of information on the duration of postoperative chemotherapy.
Statistical analysis
Comparisons between the two groups were appropriately performed with the Student’s t test or Mann–Whitney U test for continuous variables and the chi-squared or Fisher’s exact test for categorical variables. Kaplan–Meier curves were estimated for post-recurrence survival in patients with ROC. Univariate and multivariate logistic regression analyses were used to assess odds ratio (OR) for post-recurrence survival of 5 years or longer. Significance was set as two-sided with a p value < 0.05. All statistical analyses were conducted using IBM SPSS Statistics, Version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of patients
In our study cohort, there were 5264 patients with malignant ovarian tumor including malignant and borderline tumor. Among them, 3548 patients were epithelial ovarian cancer. We collected information on patients with malignant ovarian tumors and finally 657 of them that developed ROC were included in the study population. The median survival of the cohort was 22.7 [95% confidence interval (CI) 20.0–25.5] months and estimated 5- and 10-year post-recurrence survival rates were 23.2 and 12.5%, respectively (Fig. 2A). Sixty-eight (10.4%) patients were LTSROC while 399 (60.7%) were STSROC. Among LTSROC, 26 (38.2%) patients were NED and 42 (61.8%) were AWD (Fig. 2B). The baseline characteristics of LTSROC and STSROC are shown in Table 1. Age at the initial diagnosis showed the same distribution, whereas that at the diagnosis of recurrence was higher among LTSROC than STSROC. No significant differences were observed in the stage or histology distribution between the groups. More patients in STSROC than in LTSROC had a diagnosis of positive ascites cytology and remaining residual tumors. The proportion of complete-staging lymphadenectomy and postoperative chemotherapy did not significantly differ between the groups.
Survival after the recurrence of ovarian carcinoma and the proportion of long-term survivors in the study cohort. A Kaplan–Meier curves for post-recurrence survival in patients with ovarian carcinoma are shown. p values were estimated by the Log-rank test. B The proportions of long-term survivors, short-term survivors, and censored patients are shown. Among long-term survivors, the proportions of those with no evidence of disease and alive with disease were also categorized
Factors associated with long-term survival in ROC
In the univariate analysis, negative ascites cytology (OR 2.174; 95% CI 1.286–3.675; p = 0.004), remaining residual tumors at initial surgery (OR 0.537; 95% CI 0.315–0.917; p = 0.023), and RFI of 1 year or longer (OR 3.212; 95% CI 1.752–5.888; p < 0.001) were identified as factors associated with long-term survival. In the multivariate analysis, negative ascites cytology odds (OR 1.865; 95% CI 1.026–3.393; p = 0.041) and RFI of 1 year or longer (OR 2.896; 95% CI 1.546–5.425; p < 0.001) were also identified as independent factors associated with LTSROC. Other factors, including age, staging at the initial diagnosis, the level of CA-125, complete-staging lymphadenectomy, remaining residual tumors at initial surgery, postoperative chemotherapy, and the site of recurrence, were not associated with LTSROC (Table 2).
RFI and long-term survival in clinical backgrounds
Since RFI was identified as the strongest factor associated with long-term survival, we examined the distribution of RFI in each clinical background. RFI was longer in LTSROC than in STSROC (Fig. 3A). Among LTSROC, patients with NED had a significantly longer RFI than those with AWD (Fig. 3B). Regarding the status of ascites cytology, an independent factor of long-term survival, RFI was longer in LTSROC than in STSROC among patients with positive ascites cytology, but was not significantly different in those with negative ascites cytology (Fig. 3C). In stages I to III, RFI was slightly longer in LTSROC and the difference was significant for stage III (Fig. 3D). Regarding histology, RFI was also slightly longer in LTSROC than in STSROC and the difference was significant for serous carcinoma (Fig. 3E).
Plot of the recurrence-free interval of each clinical background. Difference between long-term survivors and short-term survivors in all patients (A), no evidence of disease and alive with disease in long-term survivors (B), and long-term survivors and short-term survivors in the status of ascites cytology (C), stage (D) and histology (E)
Treatment and outcomes of patients with NED and AWD in LTSROC
We assessed the clinical features of LTSROC in 46 patients with NED and AWD and sufficient data. No significant differences were observed in background information between the NED and AWD groups. More than 50% of LTSROC presented with stage III tumors at the initial diagnosis. In addition, the most common histology of LTSROC was serous carcinoma; however, other types were also confirmed. Approximately 50% of LTSROC underwent the complete operation. Regarding the site of recurrence, most patients developed peritoneal tumors. Approximately 80% of LTSROC presented with a solitary recurrent tumor. Furthermore, more than 50% of LTSROC underwent tumor debulking surgery for the first recurrent tumor with or without chemotherapy (Table 3).
Discussion
In the present study, RFI of 1 year or longer and negative ascites cytology in the initial surgery were identified as independent predictive factors associated with LTSROC. Additionally, the majority of LTSROC presented with solitary recurrent tumors, with more than 50% undergoing secondary debulking surgery. This was one of the largest retrospective cohort studies to analyze the characteristics of LTSROC under the central pathological review system. The present results will be of benefit for patients with OC when selecting therapeutic options to achieve longer survival even after tumor recurrence.
RFI from initial surgery to the development of recurrent tumors was identified as the strongest factor predicting LTSROC. This result was expected from the literature, which described progression-free interval (PFI) as a prognostic factor for survival outcomes in OC patients [12]. One reason for this stems from acquired resistance to platinum agents in ROC, which is generally defined as recurrence after less than 6 months of PFI [13]. In the present study, although we selected 1 year as the cut-off value for RFI because of a lack of information on the duration of postoperative chemotherapy, which was distinct from the common definition, the present result appeared to also reflect longer RFI being associated with long-term survival in ROC. On the other hand, PFI was significantly different between LTSROC and STSROC in the subgroups of positive ascites cytology, stage III, and serous histology. Based on these results, we need to re-recognize ROC not as one etiology, but as an individual tumor arising from each clinical background of initial OC.
The status of ascites cytology in the initial surgery was also found to influence long-term survival even after tumor recurrence in the univariate analysis. This result indicates that the presence of tumor cells in ascites reflected a different feature of progression from the conventional staging. We previously suggested that positive ascites cytology significantly deteriorated the overall survival of patients, even those with stage III OC [14]. We need to recapture the significance of positive ascites cytology and elucidate the underlying biological and oncological mechanisms leading to the poor prognosis of OC patients.
Throughout the evaluation of LTSROC, we revealed that approximately 80% of LTSROC with both NED and AWD presented with solitary recurrent tumors, while 50% underwent secondary debulking surgery for ROC. This result was consistent with the recent findings of the DESKTOP III trial, which demonstrated that cyto-reductive surgery followed by chemotherapy resulted in longer overall survival than chemotherapy alone for platinum-sensitive ROC [15]. Consequently, secondary cyto-reductive surgery was important for pursuing long-term survival in ROC. In contrast, the remaining 50% of LTSROC achieved NED or AWD with chemotherapy and/or radiation, which suggested that the rationale for long-term survival cannot be explained by one reason alone. Since the present study did not directly evaluate the effects of therapeutic interventions on the prognosis of ROC, a robust conclusion cannot be reached, and, thus, the results obtained need to be verified in future studies.
The strength of the present study was that all data were obtained from multiple institutions under the central pathological review system. On the other hand, limitations included the small sample size number because of the rarity of LTSROC. Besides, in our study cohort, the genetic status may have affected responses to treatment in patients with ROC and their post-recurrence survival. Unfortunately, we did not accumulate genetic information for our current dataset, which needs to be investigated to identify the predictive factors for LTSROC in the near future. In addition, a censored population accounted for approximately 30% of the original cohort, which may affect the significance of the results obtained. Since the factors that excluded these censored cases depended only on the time of observation, the effect of bias by excluding these cases seemed to be small. However, the present results need to be validated in future studies with the accumulation of more clinical and genetic data on LTSROC.
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33
Kajiyama H, Suzuki S, Yoshihara M et al (2019) Endometriosis and cancer. Free Radic Biol Med 133:186–192
Eisenkop SM, Friedman RL, Wang HJ (1998) Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol 69:103–108
Friedlander M, Butow P, Stockler M et al (2009) Symptom control in patients with recurrent ovarian cancer: measuring the benefit of palliative chemotherapy in women with platinum refractory/resistant ovarian cancer. Int J Gynecol Cancer 19(Suppl 2):S44–S48
Tokunaga H, Mikami M, Nagase S et al (2021) The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. J Gynecol Oncol 32:e49
Ozols RF (2006) Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol 33:S3-11
Hilal Z, Schultheis B, Hartmann F, et al (2016) What Characterizes Long-term Survivors of Recurrent Ovarian Cancer? Case report and review of the Literature. Anticancer Res 36:5365–5371.
Iwase H, Takada T, Iitsuka C et al (2015) Clinical features of long-term survivors of recurrent epithelial ovarian cancer. Int J Clin Oncol 20:143–149
Chen VW, Ruiz B, Killeen JL et al (2003) Pathology and classification of ovarian tumors. Cancer 97:2631–2632
Zeppernick F, Meinhold-Heerlein I (2014) The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet 290:839–842
Suzuki S, Kajiyama H, Shibata K et al (2008) Is there any association between retroperitoneal lymphadenectomy and survival benefit in ovarian clear cell carcinoma patients? Ann Oncol 19:1284–1287
Markman M, Rothman R, Hakes T et al (1991) Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9:389–393
Davis A, Tinker AV, Friedlander M (2014) “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol 133:624–631
Yoshihara M, Emoto R, Kitami K et al (2021) A large-scale multi-institutional study evaluating prognostic aspects of positive ascites cytology and effects of therapeutic interventions in epithelial ovarian cancer. Sci Rep 11(1):15154
Harter P, Sehouli J, Vergote I et al (2021) Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med 385:2123–2131
Acknowledgements
We sincerely appreciate the collaboration of the members of Tokai Ovarian Tumor Study Group-affiliated institutions for data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshihara, M., Mogi, K., Kitami, K. et al. Who are the long-term survivors of recurrent ovarian carcinoma?: a retrospective analysis of a multicenter study. Int J Clin Oncol 27, 1660–1668 (2022). https://doi.org/10.1007/s10147-022-02214-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02214-9