Abstract
Objective
To compare the efficacy and safety of fentanyl iontophoretic transdermal system (ITS) with morphine intravenous patient-controlled analgesia (IV PCA) for pain management following gynecologic surgery.
Method
A subgroup (n = 275) of gynecologic surgery patients from a randomized study (N = 636) of patients treated with fentanyl ITS or morphine IV PCA was analyzed. The main efficacy endpoint was the patient global assessment (PGA) of the method of pain control (first 24 h).
Result
In gynecologic surgery patients, PGA success ratings (excellent/good) were statistically equivalent (fentanyl ITS, 84.8%; morphine IV PCA, 83.9%; 95% confidence interval: -7.7%, 9.4%) based on the prespecified equivalence criterion of 10% for the entire study population. Pain intensity at 3 h (P = 0.296), discontinuations due to inadequate analgesia (P = 0.148), and percentages of patients requesting supplemental opioids in the first 3 h (P = 0.524) were similar.
Conclusion
The two modalities were therapeutically equivalent for pain management in these gynecologic surgery patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Opioids are a mainstay of analgesic therapy for moderate-to-severe pain following major gynecologic surgery. Potent opioids such as fentanyl, morphine, hydromorphone, and meperidine are commonly used in this setting and are usually administered intermittently by the intramuscular (IM) or intravenous (IV) route. In many institutions, nurse-administered IM opioid injections are increasingly being replaced by the use of different forms of patient-controlled analgesia (PCA), predominantly that of IV PCA, which results in prompt and more individualized therapy with greater patient satisfaction [7].
IV PCA allows patients to self-administer a predetermined opioid dose at appropriate intervals and to titrate analgesia to their perceived level of pain by activating a manually programmed electronic pump that delivers the analgesics through an IV catheter. Although IV PCA has resulted in dramatic improvements in the quality of postoperative pain management, limitations to this delivery method do exist. The IV PCA system requires technical expertise and training of the nursing staff to avoid potential programming errors, as well as periodic evaluation by the nursing staff for problems arising from IV line occlusion and catheter infiltration [7, 13, 20]. Patient deaths have been documented as a result of programming errors associated with IV PCA [20]. Additionally, the IV PCA pumps require periodic maintenance and replacement by biomedical engineering, and while most opioids are available in prefilled cartridges, some require cartridges to be filled by the pharmacist prior to dispensing, resulting in increased pharmacy workload.
Despite the improvements in analgesic therapy afforded by IV PCA and the ongoing publication of pain management guidelines [1, 11], studies indicate that postoperative pain continues to be undermanaged [2, 16]. To address the technical limitations of IV PCA and to further improve postoperative pain management, a preprogrammed, noninvasive, fentanyl HCl iontophoretic transdermal system (fentanyl ITS; IONSYS™, Ortho-McNeil, Inc., Raritan, NJ, USA) was developed. Fentanyl is a commonly used opioid, and its use in the treatment of postoperative pain via IV PCA has been demonstrated over a wide range of doses [8, 9, 12]. Its high lipid solubility and highly ionized state make fentanyl well suited for transdermal delivery [15]. Iontophoresis is the process by which ionized drugs are delivered across intact skin using an electrical field [22].
The fentanyl ITS is self-contained and self-adhesive, and it is applied to the patient’s upper outer arm or chest. The fentanyl ITS delivers 40 μg fentanyl (over 10 min) from a hydrogel reservoir using an imperceptible, low-intensity (170 μA) electrical field. Drug delivery is initiated when the patient double-clicks the recessed on-demand dosing button. The delivery of each dose is indicated by a beep and a red light-emitting diode that turns off following the end of the dose. When queried, the approximate number of doses delivered is displayed as a series of light flashes. If a system error occurs, it is indicated by audible cues. The safety mechanism will only allow the system to deliver fentanyl for 24 h or 80 doses, whichever occurs first, and it will not respond to additional medication requests during the 10-min dose delivery period. For pain relief beyond 24 h or 80 doses, a new system may be applied. Fentanyl ITS is approved for a maximum of 72 h of therapy.
The efficacy of the fentanyl ITS as a method of pain control has been shown to be equivalent to that of a standard regimen of morphine IV PCA in a large population of adult male and female patients who underwent a wide variety of major abdominal, orthopedic, or thoracic surgical procedures [21]. The objective of the current analysis was to evaluate the efficacy and safety of the fentanyl ITS versus morphine IV PCA in a specific female subgroup of that larger study population [21] who underwent gynecologic surgery. More than one million gynecologic surgeries were performed in the United States in the year 2002, with more than 6,69,000 of these being hysterectomy [19]. Therefore, this subgroup analysis of the larger study population will provide a useful, direct comparison of patient-controlled postoperative pain management strategies following a common, specific class of surgical procedures in female patients.
Materials and methods
Study description
This was a prospective, randomized, multicenter, open-label, active-controlled study that compared the efficacy and safety of the fentanyl ITS with morphine IV PCA for the management of postoperative pain among patients following major abdominal, orthopedic, or thoracic surgery [21]. The duration of patient follow-up in this study was 72 h. The study was conducted between September 2000 and March 2001 at 29 US and 4 Canadian hospitals. Study protocols were approved by the institutional review board or research ethics board of each participating center or by an independent centralized ethics review board. Written informed consent was obtained from each patient prior to enrollment in the study.
Patients
Patients were at least 18 years of age and were American Society of Anesthesiologists (ASA) physical status I, II, or III. Patients were excluded if they had received long-lasting regional analgesic or intrathecal opioids intraoperatively, were expected to have postoperative regional analgesia, or would require additional surgery within 36 h. Patients were also excluded if they had received opioids other than morphine, fentanyl, sufentanil, alfentanil, or more than 50 mg of meperidine intraoperatively or postoperatively. Patients who were intubated at final screening assessment and those with a history of recent opioid dependence or known or suspected opioid tolerance were excluded. Patients with active local or systemic skin disease that would preclude the application of the fentanyl ITS system, pregnant patients, and those with coexisting medical conditions that would interfere with study procedures were also not enrolled in the study.
Treatment modalities
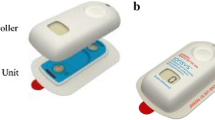
The fentanyl ITS (Fig. 1) was supplied by the sponsor. The fentanyl ITS delivered a 40-μg dose of fentanyl over a 10-min period each time the system was activated by the patient and was replaced after 80 doses or once every 24 h, whichever occurred first (up to 72 h). The decision to use a 40-μg fentanyl dose was based on the results of a clinical trial of fentanyl IV PCA demand doses carried out by Camu et al. [5], which established this dose as optimal in terms of both safety and efficacy.
The fentanyl HCl iontophoretic transdermal system (fentanyl ITS). Figure is reproduced with permission [6]
The IV PCA pumps used to administer morphine in this study were the standard pumps used by the individual institutions. Each IV PCA pump was programmed by the hospital staff to deliver a 1-mg bolus dose of morphine upon system activation by the patient, followed by a 5-min lockout period between doses, with a limit of 10 doses per hour. The morphine regimen used in this study was based on earlier work that established this regimen to be both efficacious and safe and is one that is commonly used for postoperative pain management [14]. Like the fentanyl ITS, the standard IV PCA pumps communicated system errors via audible cues [10].
Study protocol
Immediately following surgery, patients were admitted to the postanesthesia care unit (PACU) and titrated to comfort with IV doses of opioids. The choice of opioid, which could include morphine, fentanyl, sufentanil, or alfentanil, was at the discretion of the attending physician. Once patients had been in the PACU for at least 30 min, were stable and awake, and were comfortable, they were randomized 1:1 to either the fentanyl ITS or morphine IV PCA treatment groups using computer-generated random numbers for all patients regardless of the center. An interactive voice randomization system [4] prevented the investigators and their staff from knowing the next treatment assignment. During the first 3 h of treatment, supplemental analgesia was provided on request in the form of IV bolus doses of fentanyl or morphine for patients in the fentanyl ITS or morphine IV PCA groups, respectively.
Efficacy and safety endpoints
A 4-point rating scale known as the patient global assessment (PGA) of the method of pain control (excellent, good, fair, or poor) was recorded at 24, 48, and 72 h (final endpoint of study) or upon withdrawal from the study, whichever occurred first. The primary efficacy endpoint for this study was the PGA in the first 24 h. Pain intensity scores were measured on a visual analog scale (VAS) that ranged from 0 mm (no pain) to 100 mm (worst possible pain). A vertical mark was recorded directly by the patient or indicated by the patient to the investigator’s staff. Pain intensity scores, vital signs, oximetry, and the number of doses delivered were recorded at 0, 0.5, 1, 2, 3, 4, 6, and 8 h and every 4 h thereafter, up to 72 h. Patients could withdraw from the study at any time; reasons for withdrawal (e.g., inadequate analgesia, adverse event, withdrawal of consent, technical failure, protocol violation) prior to completion of 72 h of treatment were recorded and tabulated. Vital signs and adverse events were monitored and recorded throughout the study at specified times.
Statistical analyses
Analyses presented in this paper pertain to the subgroup of patients who had gynecologic surgeries in this study. This subgroup of patients is part of the overall intent-to-treat (ITT) population in the study, which was defined as all randomized patients who received the fentanyl ITS or morphine IV PCA and completed a PGA. In the case of patient withdrawal from the study, PGAs recorded at that time were carried forward to the next 24-h endpoint. If no PGA rating was recorded for a patient, a rating of poor was assumed for statistical analyses.
The primary efficacy endpoint in the main study was analyzed by constructing a two-sided 95% confidence interval (CI) for the difference in success rates between treatment groups, with success defined as a rating of excellent or good on the 4-point scale of the 24-h PGA. Therapeutic equivalence was established if the 95% CI of the difference in success rates fell within ±10%. Additional between-group analyses included the percentage of patients withdrawing from the study due to inadequate analgesia, pain intensity scores during the first 24 h of treatment, and the number of requests for supplemental opioids during the first 3 h of treatment. To compare demographic and clinical variables between treatment groups, the two-sample t-test was used for the analysis of numerical data, and the chi-square (χ 2) test was employed for categorical data. For testing the association between the ordered multiple categories of the PGA responses between the two treatments, a Wilcoxon rank sum test (giving a P value based on an asymptotic method) was used. The number of doses administered to patients in the fentanyl ITS group was estimated by using five times the number of light flashes minus two, since each light flash represented one–five doses (e.g., two flashes equaled six–ten doses).
Results
Of the 636 patients who enrolled in the original study, 275 underwent gynecologic surgery and were included in this subgroup analysis. All analyses were performed on the ITT population within the gynecologic subset of patients, which included 138 patients in the fentanyl ITS group and 137 patients in the morphine IV PCA group (Fig. 2). The most common surgery types in the gynecologic subset of patients were hysterectomy, myomectomy, and salpingo-oophorectomy. Patients who received the fentanyl ITS received treatment for a significantly longer amount of time than patients who received morphine IV PCA [mean (SEM), 37.2 (1.04) hours vs 32.2 (1.11) hours, respectively; P = 0.007]. Demographic variables were similar between the two treatment groups (Table 1). All patients were females with an average age of 45 years. The population was predominantly white.
The main efficacy endpoint for this subgroup analysis was the PGA of the method of pain control during the first 24 h of treatment (Table 2). The fentanyl ITS and morphine IV PCA were therapeutically equivalent, with 84.8% of fentanyl ITS patients and 83.9% of morphine IV PCA patients considering their method of pain control to be excellent or good (between-group difference, 0.8%; 95% CI, -7.7%, 9.4%; P = 0.848). The overall distribution of patients giving ratings of excellent, good, fair, or poor was not statistically different between the two treatment groups (P = 0.161). The success ratings on the PGA were also comparable among those gynecologic patients remaining in the study (observed cases) at 48 (94.9% vs 87.8%) and at 72 h (93.9% vs 89.5%) who received fentanyl ITS compared with those treated with morphine IV PCA, respectively (Table 3).
The mean pain intensity scores (measured on a 100-mm VAS) were similar between treatment groups at each measured time point (Fig. 3a). The mean of the last-recorded pain scores was not significantly different between patients in each treatment group who received at least one dose of study medication (28.4 for the fentanyl ITS versus 26.4 for morphine IV PCA; P = 0.477). In addition, as shown in Fig. 3b, the distribution of pain intensity scores at 3 h and at the last-recorded time point was similar between treatment groups (P = 0.296 and P = 0.446, respectively). The total number of patients requesting supplemental IV opioids within the first 3 h of treatment was comparable between treatment groups (18.1% of fentanyl ITS patients vs 21.2% of morphine IV PCA patients; P = 0.524).
Pain intensity scores over time and distribution of pain intensity scores. Pain intensity scores were measured on a 100-mm visual analog scale (VAS), with 0 representing “no pain” and 100 representing “worst possible pain.” a Pain intensity scores over the 24-h treatment period were similar between treatment groups. b The distribution of pain intensity scores at 3 h and at the last pain measurement time point was similar between treatment groups
The number of patients who discontinued the study for any reason was not significantly different between the two groups [27 (19.6%) and 29 (21.2%) patients in the fentanyl ITS and morphine IV PCA groups, respectively; P = 0.741; Table 4]. In addition, the number of patients who completed at least 24 h of treatment was similar between the fentanyl ITS and morphine IV PCA groups [118 (85.5%) and 121 (88.3%), respectively; P = 0.592]. There was no significant difference between treatment groups in the number of patient withdrawals due to inadequate analgesia [12 (8.7%) and 6 (4.4%) in the fentanyl ITS and morphine IV PCA groups, respectively; P = 0.148]. In contrast, significantly more patients in the morphine IV PCA group withdrew from the study due to “other” reasons [12 (8.8%) morphine IV PCA patients versus 4 (2.9%) fentanyl ITS patients; P = 0.038], with one third of morphine IV PCA patients withdrawing due to problems with IV infiltration. The most common “other” reason for patient withdrawal in either treatment group was the patient-requested or investigator-initiated addition of analgesic medications other than those specified in the study protocol (Table 4).
The most commonly observed treatment-related adverse events in both treatment groups were nausea, headache, and pruritus (Table 5). Treatment-related adverse events were similar between treatment groups and led to study discontinuation in 6.5% of patients in the fentanyl ITS group and 5.1% of patients in the morphine IV PCA group (P = 0.617; Table 4). Due to the nature of the delivery system, only patients in the fentanyl ITS group experienced application-site reactions (9.4% of patients), the majority of which involved mild erythema, itching, and vesicle formation (Table 5). None of the application-site reactions resulted in discontinuation from the study.
Discussion
Gynecologic surgery is a common operation in the United States, where more than one million procedures were performed in the year 2002 [19]. Recent efforts to improve postoperative pain management, such as the development of acute pain services, which combine patient education with frequent pain assessments and the use of adjunctive treatments, such as nonsteroidal anti-inflammatory drugs (NSAIDs), have resulted in decreased postoperative opioid requirements in gynecologic patients [3, 16, 18]. IV PCA has resulted in dramatic improvements in the quality of postoperative pain management; however, limitations of the delivery method do exist, including equipment-related issues and the potential for programming errors and needlestick injuries.
This subgroup analysis of the gynecologic surgery patients suggests that the fentanyl ITS is therapeutically equivalent to a standard regimen of morphine IV PCA, as measured by the PGA of the method of pain control during the first 24 h of treatment. Additional efficacy measures, including mean pain intensity scores and withdrawals from the study due to inadequate analgesia, were also comparable between the fentanyl ITS and morphine IV PCA groups, supporting the assessment of therapeutic equivalence. It should be noted that discontinuation from the study due to “other” reasons, with one third being IV infiltration problems, was significantly higher in the morphine IV PCA group compared with the fentanyl ITS group. Adverse events were comparable between the two groups, with the most frequently reported events being those that are commonly associated with opioid administration, namely nausea, headache, and pruritus.
The results of this subgroup analysis were similar to those observed with the overall study population and described by Viscusi et al. [21]. Interestingly, higher percentages of patients who received fentanyl ITS or morphine IV PCA in this analysis reported success ratings on the PGA of the method of pain control during the first 24 h (84.8% vs 83.9%, respectively) compared with patients in the overall population (73.7% vs 76.9%, respectively). In addition, rates of discontinuation due to inadequate analgesia were lower in this subpopulation of patients who received fentanyl ITS or morphine IV PCA following gynecologic surgery (8.7% vs 4.4%, respectively) compared with rates that were observed with the overall population (15.3% vs 10.9%, respectively). Overall, the findings from this subgroup analysis support the conclusion of the original study that fentanyl ITS provides effective pain control comparable to that provided by a standard regimen of morphine IV PCA following major surgery, such as gynecologic surgery.
Studies have shown that the use of IV PCA results in earlier discharge from the hospital, reduced morbidity, enhanced analgesia, and decreased consumption of opioids in patients following gynecologic surgery compared with IM injection [17, 23]. However, many studies have identified potentially serious adverse events associated with IV PCA, including IV line occlusion, IV infiltration of the surrounding tissue, and programming errors [7, 13, 20]. In the current subgroup analysis, IV line infiltrations led to study withdrawal in four patients in the morphine IV PCA treatment group, underscoring one of the problems associated with IV PCA administration. This was not an issue with the fentanyl ITS, as its needle-free design addresses this problem by providing effective drug delivery without the need for IV access.
Although favorable results were observed with the fentanyl ITS in this subgroup of gynecologic surgery patients, limitations are noted in the overall study design. One is that the study was not blinded, since this would require both the fentanyl ITS and the IV PCA pump to be attached simultaneously to patients to simulate drug delivery from either system, which could be confusing to patients and potentially affect patient assessment of either drug delivery method. Under this scenario, the logistical consideration of patients being required to press two dosing buttons simultaneously for drug delivery also limited this approach. In addition, supplemental analgesia was prohibited after the first 3 h of treatment in the study, which may not be representative of the true nature of postoperative pain management in a clinical setting, as adjunctive therapies, such as NSAIDs, and opioid analgesia are commonly used.
No pharmacoeconomic analyses have been performed to date with fentanyl ITS. However, it is expected that the self-contained, preprogrammed system will save time for health care providers and subsequently reduce labor costs, since the system eliminates tasks associated with assembly and programming and more importantly may reduce the incidence of avoidable medication errors that are associated with other PCA modalities.
In conclusion, the fentanyl ITS provides pain relief and a safety profile comparable to that of morphine IV PCA following gynecologic surgery. The noninvasive nature of drug delivery by the fentanyl ITS avoids the potential complications of IV therapy, such as catheter infiltration, which in this study resulted in several patients receiving morphine IV PCA discontinuing treatment. Future studies should examine the use of the fentanyl ITS for acute pain management in other therapeutic areas.
References
American Society of Anesthesiologists Task Force on Acute Pain Management, Ashburn MA, Caplan RA, Carr D, Connis R, Ginsberg B, Green C, Lema M, Nickinovich DG, Rice LJ (2004) Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 100:1573–1581
Apfelbaum JL, Chen C, Mehta SS, Gan TJ (2003) Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 97:534–540
Bardiau FM, Braeckman MM, Seidel L, Albert A, Boogaerts JG (1999) Effectiveness of an acute pain service inception in a general hospital. J Clin Anesth 11:583–589
Byrom B (2002) Using IVRS in clinical trial management: interactive voice response systems can work for project managers as an inventory management tool, a real-time project information tool, and a subject recruitment tool. Appl Clin Trials 11:36(5)
Camu F, Van Aken H, Bovill JG (1998) Postoperative analgesic effects of three demand-dose sizes of fentanyl administered by patient-controlled analgesia. Anesth Analg 87:890–895
Chelly JE (2005) An iontophoretic, fentanyl HCl patient-controlled transdermal system for acute postoperative pain management. Expert Opin Pharmacother 6:1205–1214
Etches RC (1999) Patient-controlled analgesia. Surg Clin North Am 79:297–312
Glass PS, Estok P, Ginsberg B, Goldberg JS, Sladen RN (1992) Use of patient-controlled analgesia to compare the efficacy of epidural to intravenous fentanyl administration. Anesth Analg 74:345–351
Howell PR, Gambling DR, Pavy T, McMorland G, Douglas MJ (1995) Patient-controlled analgesia following caesarean section under general anaesthesia: a comparison of fentanyl with morphine. Can J Anaesth 42:41–45
Jacox A, Carr DB, Mahrenholz DM, Ferrell BM (1997) Cost considerations in patient-controlled analgesia. Pharmacoeconomics 12:109–120
Joint Commission on Accreditation of Healthcare Organizations (2000) Pain assessment and management: an organizational approach. JCAHO, Oakbrook, IL
Lehmann KA, Heinrich C, van Heiss R (1988) Balanced anesthesia and patient-controlled postoperative analgesia with fentanyl: minimum effective concentrations, accumulation and acute tolerance. Acta Anaesthesiol Belg 39:11–23
Macintyre PE (2001) Safety and efficacy of patient-controlled analgesia. Br J Anaesth 87:36–46
Owen H, Plummer JL, Armstrong I, Mather LE, Cousins MJ (1989) Variables of patient-controlled analgesia. 1. Bolus size. Anaesthesia 44:7–10
Peng PW, Sandler AN (1999) A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 90:576–599
Salomaki TE, Hokajarvi TM, Ranta P, Alahuhta S (2000) Improving the quality of postoperative pain relief. Eur J Pain 4:367–372
Smythe M, Loughlin K, Schad RF, Lucarroti RL (1994) Patient-controlled analgesia versus intramuscular analgesic therapy. Am J Hosp Pharm 51:1433–1440
Tang J, Li S, White PF, Chen X, Wender RH, Quon R, Sloninsky A, Naruse R, Kariger R, Webb T, Norel E (2002) Effect of parecoxib, a novel intravenous cyclooxygenase type-2 inhibitor, on the postoperative opioid requirement and quality of pain control. Anesthesiology 96:1305–1309
US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (2003) National Center for Health Statistics Fast Stats, Inpatient Surgery, 2003. http://www.cdc.gov/nchs/fastats/insurg.htm. Accessed 18 October 2006
Vicente KJ, Kada-Bekhaled K, Hillel G, Cassano A, Orser BA (2003) Programming errors contribute to death from patient-controlled analgesia: case report and estimate of probability. Can J Anaesth 50:328–332
Viscusi ER, Reynolds L, Chung F, Atkinson LE, Khanna S (2004) Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA 291:1333–1341
Viscusi ER, Witkowski TA (2005) Iontophoresis: The process behind noninvasive drug delivery. Reg Anesth Pain Med 30:292–294
Wasylak TJ, Abbott FV, English MJ, Jeans ME (1990) Reduction of postoperative morbidity following patient-controlled morphine. Can J Anaesth 37:726–731
Acknowledgments
The authors wish to acknowledge the investigators who contributed participants to this subgroup analysis: Raymond L. Brewer, MD, Rose Medical Group, University City, Texas; A. John Clark, MD, FRCPC, Queen Elizabeth II Health Sciences Center, Halifax, Nova Scotia; Craig S. Curry, MD, Maine Medical Center, Portland, Maine; Robert B. Fisher, DO, University of Missouri, Columbia, Missouri; Brian Ginsberg, MD, Duke University Medical Center, Durham, North Carolina; Michael E. Goldberg, MD, Cooper Hospital, Camden, New Jersey; Daniel E. Kenady, MD, University of Kentucky Medical Center, Lexington, Kentucky; Samia Khalil, MD, University of Texas Medical School, Houston, Texas; Anthony L. Kovac, MD, University of Kansas Medical Center, Kansas City, Kansas; Cynthia Lien, MD, New York Presbyterian Hospital, New York, New York; Timothy Lubenow, MD, Rush-Presbyterian-St Luke’s Medical Center, Chicago, Illinois; Tim Melson, MD, Helen Keller Hospital, Sheffield, Alabama; Harold S. Minkowitz, MD, Memorial Hermann Memorial City Hospital, Houston, Texas; Joel L. Parlow, MD, Queens University, Kingston General Hospital, Kingston, Ontario, Canada; Lowell Reynolds, MD, Loma Linda University, Loma Linda, California; Adam Sackstein, MD, St Francis Medical Center, Trenton, New Jersey; Jeffrey H. Silverstein, MD, Mount Sinai School of Medicine, New York, New York; Daneshvari Solanki, MD, University of Texas, Galveston, Texas; Robert Steinberg, MD, PhD, Baystate Medical Center, Springfield, Massachusetts; Eugene R. Viscusi, MD, Thomas Jefferson University, Philadelphia, Pennsylvania; Barth L. Wilsey, MD, University of California, Davis, California; Joel Yarmush, MD, MPA, New York Methodist Hospital, Brooklyn, New York.
Author information
Authors and Affiliations
Corresponding author
Additional information
ALZA Corporation, Mountain View, CA, supported this study in its entirety. Dr Hewitt is an employee of Ortho-McNeil, Inc. Dr Damaraju is an employee of Ortho-McNeil Janssen Scientific Affairs, LLC.
Rights and permissions
About this article
Cite this article
Ahmad, S., Hewitt, D.J. & Damaraju, C.V. Fentanyl HCl iontophoretic transdermal system versus intravenous morphine pump after gynecologic surgery. Arch Gynecol Obstet 276, 251–258 (2007). https://doi.org/10.1007/s00404-007-0339-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-007-0339-z