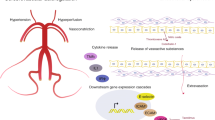
Abstract
Purpose of Review
To report a series of patients with clinical and radiological features suggestive of posterior reversible encephalopathy syndrome (PRES) related to diverse etiologies emphasizing its pathophysiological basis.
Recent Findings
Posterior reversible encephalopathy syndrome (PRES) may present with a broad range of clinical symptoms from headache and visual disturbances to seizure and altered mentation. Typical imaging findings include posterior-circulation predominant vasogenic edema. Although there are many well-documented diseases associated with PRES, the exact pathophysiologic mechanism has yet to be fully elucidated. Generally accepted theories revolve around disruption of the blood-brain barrier secondary to elevated intracranial pressures or endothelial injury induced by ischemia from a vasoconstrictive response to rising blood pressure or toxins/cytokines. While clinical and radiographic reversibility is common, long-standing morbidity and mortality can occur in severe forms. In patients with malignant forms of PRES, aggressive care has markedly reduced mortality and improved functional outcomes. Various factors that have been associated with poor outcome include altered sensorium, hypertensive etiology, hyperglycemia, longer time to control the causative factor, elevated C reactive protein, coagulopathy, extensive cerebral edema, and hemorrhage on imaging. Reversible cerebral vasoconstriction syndromes (RCVS) and primary angiitis of the central nervous system (PACNS) are invariably considered in the differential diagnosis of new cerebral arteriopathies. Recurrent thunderclap headache (TCH), and single TCH combined with either normal neuroimaging, border zone infarcts, or vasogenic edema, have 100% positive predictive value for diagnosing RCVS or RCVS-spectrum disorders. Diagnosis of PRES in some circumstances can be challenging and structural imaging may not be sufficient to distinguish it from other differential diagnostic considerations like ADEM. Advanced imaging techniques, such as MR spectroscopy or positron emission tomography (PET) can provide additional information to determine the diagnosis. Such techniques are more useful to understand the underlying vasculopathic changes in PRES and may answer some of the unresolved controversies in pathophysiology of this complex disease.
Summary
Eight patients with PRES resulting from different etiologies varying from pre-eclampsia/eclampsia, post-partum headache with seizures, neuropsychiatric systemic lupus erythematosus, snake bite, Dengue fever with encephalopathy, alcoholic liver cirrhosis with hepatic encephalopathy, and lastly reversible cerebral vasoconstriction syndrome (RCVS). Additionally, a diagnostic dilemma between PRES and acute disseminated encephalomyelitis (ADEM) was notable in one patient. Some of these patients did not have or only very transiently had arterial hypertension. PRES may underlie the clinical conundrum of headache, confusion, altered sensorium, seizures, and visual impairment. PRES need not necessarily be always associated with high blood pressure. Imaging findings may also be variable. Both clinicians and radiologists need to familiarize themselves with such variabilities
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long before the introduction of the acronym PRES, physicians linked high blood pressure to brain damage. White matter abnormalities were observed earlier in eclampsia and particularly with cyclosporine-induced leukoencephalopathy [1, 2]. In the 1980s, white matter edema on head CT was associated with hypertension. With the advent of brain MRI, the more posterior brain location was determined, and its reversibility with treatment noted [1,2,3,4,5,6, 7••]. Currently, PRES is becoming increasingly recognized, largely because of the wide availability of neuroimaging. The pathophysiology remains poorly understood, although endothelial dysfunction resulting in disruption of the BBB is recognized as the final common pathway irrespective of etiology. As a result, PRES is often considered as the definitive diagnosis in the setting of acute neurological symptoms among patients with renal failure, uncontrolled hypertension, use of cytotoxic drugs, a variety of autoimmune disorders, or eclampsia especially when focal neurological signs are not evident and patients complain of bilateral blurred vision bilaterally. Distinguishing radiological features of PRES include transient bilateral parieto-occipital T2 hyperintensities, single hemispheric watershed area involvement, and frontal sulcal area involvement [8••, 9••, 10••]. Associated hemorrhage, restricted diffusion, contrast enhancement, and arteriolar vasoconstriction may also be seen in many cases. Given sufficient time and with appropriate supportive treatment, the white matter lesions generally resolve spontaneously. However, in severe cases, PRES might cause substantial morbidity and even death [5]. Hinchey et al. [7••] used this term for the first time and described fifteen patients who were already suffering from a wide variety of medical illnesses. In this communication, we report on our experience with seven cases of PRES of various etiologies, and one with a diagnostic difficulty and provide an update review of the available literature.
Case Reports
Case 1
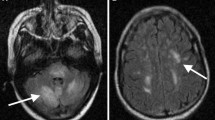
A 27-year-old woman with 32 weeks gestational age and irregular antenatal check-up was admitted to the obstetric ward with acute onset of severe holocranial headaches, visual blurring, and vomiting. Blood pressure (BP) was 170/110mm Hg. She had bilateral pedal edema and proteinuria. She had emergency cesarean section delivering a live baby who needed resuscitative measures. Following delivery, the patient had three generalized seizures. The obstetricians treated her with IV magnesium sulfate and antihypertensives as her BP climbed to 190/110 mm Hg. Glasgow Coma Score (GCS) was 10. Funduscopy showed retinal vasospasm and blotchy hemorrhages. Head CT showed bilateral symmetrical hypodensities involving the parieto-occipital regions but stopping short of the central sulci (Fig. 1). Brain MRI showed symmetrical hyperintensities in both parieto-occipital regions and in the pons on T2 weighted and FLAIR images (Figs. 2 and 3). She made a gradual recovery with restoration of vision and was discharged home after 2 weeks.
A follow-up brain MRI 3 months later showed complete clearing of the cerebral lesions but a discrete hypointensity (T1-weighted image) persisted in the pons (Fig. 4). BP was normal without anti-hypertensive drugs.
Comments
This patient had classic features of pre-eclampsia. Soon after delivery, she developed eclampsia. She also had typical features of PRES [11]. Pre-eclampsia/eclampsia is primarily a placental disorder. Both poor placentation and hyper-placentosis are associated with this condition. It is posited that either an unknown factor or villous debris from the malformed placenta reach the maternal circulation, inducing an immunological response from the mother’s system.
The maternal response consists of immunological endothelial damage with increased capillary permeability, raised fibronectin levels and electron microscopic evidence of damaged endothelium. Coagulation abnormalities [12] occur, as a result of increased activation and consumption of platelets, low antithrombin levels, abnormal prostaglandin metabolism, and in a few cases, florid disseminated intravascular coagulation (DIC). Endocrine dysfunctions include activation of the renin-angiotensin aldosterone axis, abnormal catecholamines, and abnormalities in progesterone metabolism. These pathophysiological changes lead to hypertension, edema, proteinuria, bleeding tendencies, neurological abnormalities and multiorgan dysfunction [1314••, 15].
Case 2
A 27-year-old primigravida had two generalized tonic-clonic seizures 17 days after a full term normal delivery. She had regular antenatal check-ups and her BP had always been normal. Soon after seizures, her BP was 140/100 mm Hg. Urinalysis showed trace of albumin. She had been complaining of severe headaches and bilateral visual blurring. Later, her BP came down to 120/80 mm Hg. Fundi showed optic disc swelling, retinal vessel narrowing, macular edema, and hemorrhages (Fig. 4 A and B). Brain MRI (T2 and FLAIR sequences) demonstrated patches of increased signal intensities in the white matter of frontal and parietal lobes mostly, with relatively sparing of the occipital lobes (Fig. 5). There was no MRV evidence of venous sinus thrombosis.
Comments
Judging by the association with post-partum eclampsia, the brain MR features need to be considered as PRES although anatomically the involvement was more anterior than posterior. In patients with pre-existing epilepsy, seizures may occur specially during the second trimester as a result of hemodilution causing lowering of antiseizure drug level. Some subjects prone to develop epileptic seizures may have their first seizure during pregnancy possibly related to altered hormonal balance.[13]
In the present case, the seizures seemed to be related to PRES though occurring at an uncommon site, namely the frontal sulcal regions.
Case 3
A 28-year-old woman, with no past history of seizures or pre-eclampsia, had an uneventful vaginal delivery under epidural anesthesia. She had regular antenatal check-ups and her blood pressure was normal. She slept well at night and periodic BP recordings were made which remained around 120/80 mmHg. On waking up the next morning, she had a dull headache. When her BP was re-checked, it was found to be satisfactory. Then, she became restless and soon she had a generalized tonic-clonic convulsion, which lasted for about 10 min. Immediately post-seizure, her BP was 140/90 mmHg but that settled between 120–130/70–80 mmHg within the next hour. She had no pedal edema, nor any proteinuria. Neurological examination about two hours later did not demonstrate any focal signs with normal looking fundi. EEG showed mild diffuse slowing with intermittent symmetrical runs of delta activity over both posterior brain regions. The MR image (FLAIR sequence) revealed symmetrical areas of hyperintensity involving both occipital regions (Fig. 6). There was no extension of the lesions anteriorly. The features were highly suggestive of PRES. The reversible nature of the pathology could only be demonstrated by a repeat MRI done at least 3 months later, and this could not be arranged. She remained well, with controlled BP and had no recurrence of seizures. In essence, the underlying pathology seemed to be vasogenic edema. MR angiography and venography were normal.
Comments
Radiological demonstration of PRES in a post-partum scenario with headache and seizures, almost always is pathognomonic of pre-eclampsia/eclampsia. What is unique in the present case is that the patient never had any feature to suggest pre-eclampsia or pre-existing hypertensive disease prior to the occurrence of seizure and even post-ictally. The question therefore arises whether the seizure represents occurrence of an underlying eclamptic process without any overt clinical manifestation or this represents the first occurrence of an unprovoked epileptic seizure. Occurrence of symmetrically localized vasogenic edema following a seizure of short duration would be highly unlikely. In the present case, however, there had never been any documentation of acute rise in BP nor other features of pre-eclampsia. Although the majority of patients with pregnancy-related PRES, typically present with severe hypertension, cases have been documented of patients with only mild hypertension or even normotension [13, 14••, 15]. In view of these reports and having excluded other possible causes of seizures in the immediate post-partum period, we posited that the cause of PRES and subsequent seizure was most likely related to a pre-eclamptic/eclamptic process, even though the classical features had been absent, especially acute hypertension. Furthermore, it seems that a post-partum eclamptic process without classical features should be included in the evaluation of patients presenting with post-partum headaches only but no seizures.
Case 4
A 44-year-old normotensive woman was admitted to hospital after having had her first episode of a generalized tonic-clonic seizure followed by post-ictal vomiting. On admission, she was fully conscious, and oriented. There were no abnormal neurologic signs. In particular, she had no visual disturbances. BP was 180/110 mmHg; this settled to 130/80 mmHg after about 6–8 h. EEG showed bilaterally symmetrical posteriorly dominant high amplitude (> 100 uv) delta waves. Brain MR images showed bilateral symmetrical hyperintensities exclusively restricted to the white matter of the both occipital and posterior parietal lobes (Fig. 7). Additionally, there were microbleeds on either side. Of note, she had a history of snakebite one month back and had received anti venom serum.
Comments
A major factor in the development of PRES in most patients is hypertension-related disorders like eclampsia. In the present case, the patient had not been known to be hypertensive and had no history suggestive of an eclamptic disorder during her pregnancies. A transient rise of BP was noted on admission which could be related to her having had a generalized seizure shortly before admission and other possible stressors. Her BP was normal soon after settling down in the ward. Thereafter, it remained normal without being on any antihypertensive drug. So, this transient rise in her BP is unlikely to be a cause for her PRES. In fact, her seizure was most likely caused by PRES, which is well known. The patient gave a history of a snakebite (possibly viper) 1 month back following which she was admitted to another institution. She received anti-venom serum injection, which is a routine practice. She also developed acute kidney injury (AKI) with marked oliguria for which she received hemodialysis and got better.
The question then arises—which one of these three factors caused her PRES?
A few cases of PRES following snakebite have been previously reported. Snake venom might have cytotoxic, hypotensive, neurotoxic, and anticoagulant or procoagulant effects. These snake toxins account for the local and systemic clinical manifestations of snakebite depending on the species of the snakes [16•]. While such toxins may be implicated in the pathogenesis of central nervous system (CNS) and renal manifestations, venom neurotoxins also have blocking action at the neuromuscular junction resulting in a paralytic syndrome resembling botulism. CNS effects of snakebite cannot be a direct effect of venom as the venom toxins do not cross the BBB [17•]. Both ischemic as well as hemorrhagic strokes may follow snakebites. While the anticoagulant properties of some of the toxins may explain the causation of hemorrhagic stroke, the exact pathogenetic mechanism for ischemic stroke is unknown. Potential mechanisms include toxin-induced hypercoagulability, systemic hypotension, thrombotic microangiopathy, immune-mediated vasculitis, and venom-induced endothelial damage [18, 19]. The latter may have a role in the genesis of PRES following snakebite through endothelial leakage causing vasogenic edema, or a direct toxic effect on small vessels causing cytotoxic edema or demyelination. All these processes can be reversible [20].
The polyvalent antivenom that the patient received is also not free of serious side effects and may be causally related to PRES [21].
Lastly, development of PRES following both peritoneal and hemodialysis have been reported [22, 23].
So, several factors might have contributed to this patient’s development of PRES of which the endothelial damaging effect of the venom toxins seems to be the major one.
Case 5
A 29-year-old woman was admitted with a 6-month history of gradually developing generalized weakness and body aches, abnormal behavior, generalized skin rash, joint pain, low-grade fever, and hair loss—all developing gradually over the preceding 6 months. Her relatives noticed some behavioral abnormality with steady cognitive decline. Additionally, she had intermittent headaches, visual hallucinations, depressed mood, reduced attention span, and anxiety. By the time of hospital admission, she was bed bound with moderately severe body aches, which aggravated with movements. Skin rash developed over face and was centripetal in nature, flat, non-scarring, non-itchy, and non-scaly, healed with dark pigmentation. She had small joints pain but no joint swelling or deformities. She was febrile on admission (101 °F). and a generalized maculopapular rash was present. Hair loss with similar rash over her scalp was noted. She was conscious and oriented. No obvious cognitive decline could be ascertained at bedside clinical examination. Visual acuity in both eyes was normal and she did not have any visual field deficit on confrontation. Funduscopy showed soft yellow exudates in the retina suggestive of chorioretinitis. She had grade 3/5 strength in the proximal muscles of all four limbs with intact muscle stretch reflexes and bilateral flexor plantar responses. She was normotensive.
On the second day of admission, she had a generalized tonic-clonic seizure following which she remained unarousable with a GCS varying between 5 and 6. There were no meningeal signs and she had bilateral Babinski signs.
Routine laboratory examinations showed mild anemia, normal leucocyte, and platelet counts, raised ESR but normal CRP, raised LDH and CK levels. Immunological parameters included the following: ANA (Hep-2 cell line) 1:160 dilution: positive; SSA: positive; dsDNA: strongly positive; ribosomal-P-protein: strongly positive. EEG showed post-ictal changes. EMG demonstrated a myopathic pattern. Nerve conduction studies showed changes compatible with early axonopathy in the lower limbs. Brain MRI without contrast showed bilateral near symmetrical white matter hyperintensities in the parieto-occipital regions and scattered hyperintensities in both fronto-parietal regions suggestive of PRES. (Fig. 8). She received a pulse dose of methylprednisolone intravenously daily for 5 days, and then placed on tapering dose of corticosteroids. Subsequently, she was on oral prednisolone, hydroxychloroquine, and monthly doses of cyclophosphamide. After about 3 months, she had significant improvement in her motor functions and cognition and did not have any further seizures. Repeat brain MRI showed resolution of the parieto-occipital hyperintensities.
Comments
This young woman presented with a relatively short history of fever, general weakness, joint pain without swelling/deformity, muscle weakness, and skin rash especially on exposure to sun. All these features in association with strongly positive ANA and dsDNA suggested a diagnosis of SLE. Furthermore, she had neurological and psychiatric issues, and hence, the complete diagnosis should be neuro-psychiatric SLE (NPSLE) [24••, 25].
The next question is whether she could be labeled as having an overlap syndrome. Patients with mixed connective tissue disorder (MCTD) classically have few features of SLE and few features of systemic sclerosis. She had none of the latter. The immunological markers of MCTD are high titers of autoantibodies to ribonucleoprotein (RNP or more specifically U1-RNP) and absence of anti-Sm and anti-DNA [26] antibodies. Such patients also have a high incidence of pulmonary hypertension. This patient did not have any such features. However, she did have electrographically proven myopathy, raised muscle enzymes, and myoglobinuria—all pointing towards an inflammatory muscle disease. Furthermore, her scalp skin biopsy showed features similar to dermatomyositis. Hence, taking all these into account, while she might not have a classic picture of MCTD, she may be diagnosed as having an overlap syndrome with neuro-psychiatric symptoms. The non-contrast MR imaging of the brain showed symmetrical extensive hyper-intensities in the subcortical white matter, especially in the posterior regions, making a somewhat gyriform appearance, in T2-weighted and FLAIR sequences . This pattern is highly suggestive of PRES developing in a case of NPSLE [27, 28•]. PRES associated with SLE may not be totally reversible, especially if associated with microhemorrhages and microinfarcts. There indeed are important differentials of PRES to consider in patients with SLE. These are infections, microinfarcts, venous sinus thrombosis, and lastly demyelination [29, 30, 31••, 32, 33].
Case 6
A 68-year-old-woman without any preexisting comorbidity, presented with history of fever for 5 days followed by altered sensorium for last 2 days and generalized tonic-clonic seizures in the 24 h prior to admission. On admission, she was febrile and tachypneic. Blood pressure was 80/60 mmHg. GCS was 7/15. She had bilateral Babinski signs.
Routine hematological studies showed thrombocytopenia (platelets 36,000/cmm). Biochemical profile demonstrated deranged hepatic and renal profiles. Dengue NS1 antigen and IgM antibody were positive. Chest X-ray and abdominal ultrasound were normal. Cerebrospinal fluid (CSF) protein content was elevated (80 mg%) with normal glucose and cell count.
Her sensorium remained unaltered even after 5 days with conservative and supportive treatment. The possibility of dengue encephalopathy/encephalitis was considered. Brain MRI showed (Fig. 9) bilateral symmetrical hyperintensities in the parieto-occipital subcortical white matter. There was no diffusion restriction, no contrast enhancement, and no GRE blooming. MRI findings were consistent PRES.
Supportive treatments were continued and she showed gradual improvement in her sensorium over the next 2 days. However, on regaining her senses, she complained of blurring of vision in both eyes. Visual acuity was down to 6/36 in both eyes. Fundoscopy and pupillary reactions were normal, suggesting the visual loss to be of cortical in origin. A repeat brain MRI (Fig. 10) was done 2 weeks later showed complete resolution of the hyperintense lesions. Her vision improved to 6/12 at the time of hospital discharge.
Comments
In this patient, the clinical and radiological pictures are vastly different from what is generally noted with dengue virus neuroinvasion. The symmetrical T2 hyperintensities seen in the parieto-occipital cortices, which ultimately disappeared with clinical improvement, were due to PRES rather than dengue encephalitis. Furthermore, bilateral cortical visual loss could be demonstrated when her sensorium improved. This is not described in cases of dengue encephalitis [34, 35].
The other differential related to dengue infection would be dengue-related ADEM. This is an immune mediated syndrome with features like multifocal scattered white matter and grey matter lesions seen radiologically with an abnormal CSF with raised protein content, pleocytosis and often oligoclonal bands [36]. ADEM generally responds to high dosage of corticosteroids. This patient had clinical improvement as well as reversibility of the lesions only with supportive treatment without any use of steroids suggesting the diagnosis of PRES rather than ADEM [37••].
In dengue, two major mechanisms seem to be operative in the genesis of PRES. Vasogenic edema may occur due to failure of autoregulation of cerebral blood flow, either dysregulated vasodilatation or vasoconstriction. Additionally, toxin-induced damage to the endothelium causing cytotoxic edema may also occur. This leakage may be in the brain causing PRES like changes as well as generalized cerebral edema causing dengue encephalopathy [38, 39]. Furthermore, fluid accumulation in various serous cavities of the body may lead to loss of intravascular fluid volume and dengue shock syndrome. Platelet activation and secretion of platelet activating factor (PAF) and release of nitrous oxide are other implicated pathologic mechanisms [40, 41].
Case 7
A 56-year-old man with alcoholic liver cirrhosis with portal hypertension was admitted as an emergency after being found less responsive following an episode of upper gastrointestinal bleeding presumably from esophageal varices. The bleeding varices were treated with sclerotherapy. He remained with a GCS of 8/15 with a diagnosis of hepatic encephalopathy (HE) grade 2. He then had two episodes of generalized tonic-clonic seizures in quick succession. He was started on parenteral levatiracetam. Liver functions were grossly abnormal with more than 100% increase in serum levels of the transaminases and alkaline phosphatase. Arterial ammonia raised to 180 μg/dL. INR was 3.4. He was receiving the usual treatment for HE along with mannitol.
EEG showed generalized slowing of the background consisting mostly of theta rhythm and high amplitude (> 100 uv) delta waves. No triphasic waves or any electrographic seizure discharges were recorded. Brain MRI is depicted below (Fig. 11)
Such MRI findings are consistent with PRES [42••], though slightly atypical in the sense that the lesions did not involve the occipital regions. Also, the reversibility of the lesions could not be demonstrated here as the causative illness (namely decompensated liver cirrhosis with HE) remained incurable.
Comments
The imaging features are slightly atypical but was consistent with fluid accumulation in the white matter in a symmetrical manner, though not strictly in the parieto-occipital regions. However, reversibility could not be demonstrated. Earlier, PRES had been described in patients with fulminant hepatitis and HE [43, 44]. The lesions seen in this patient’s brain imaging represents vasogenic edema remaining localized in the white matter region only. Other possible etiologies, including exaggerated immune response or cytokine release may enhance systemic endothelial activation. Alcohol per se probably had no significant role in the genesis of PRES either as the patient had abstained from alcohol for the previous 18 months or so since the diagnosis of liver cirrhosis had been made. A differential autoregulatory mechanism of cerebral blood flow between the anterior and posterior regions of the brain, related to differential autonomic innervations of the arterioles and pial vessels arising from the anterior and posterior cerebral circulations, is thought be the reason behind the posterior locations of PRES in most cases [45, 46••]. However, how relevant this hypothesis would be in relation to the present case is doubtful. Development of PRES-like lesions appeared to be the most likely reason for onset of seizures in the present case.
Case 8
A 48-year-old woman presented to the ER with history suggestive of thunderclap headache (TCH) precipitated by some unaccustomed exercise in form of carrying two bucket full of water over one flight of stairs. She was a known hypertensive and was on metoprolol 50 mg daily. At the ER, her BP was 170/100 mmHg. She was fully conscious and oriented. She had no meningeal signs. She had no visual symptoms and her fundi were normal. A plain head CT excluded subarachnoid or intracerebral bleeding. CSF was clear with no xanthochromia. She was treated with rest, antihypertensives and nimodipine because of strong suspicion of reversible cerebral vasoconstriction syndrome (RCVS) as the underlying cause. MRI brain and MRA (TOF) were done the next morning. The relevant pictures are given in Fig. 12 A and B). Headache completely settled in about 72 h, and her BP was also controlled.
A, B Case 8. A 48-year-old-woman presented with thunderclap headache precipitated by unaccustomed exercise. Intracranial bleeding was excluded. BP was 170/100 mm Hg. T2-weighted sequence MRI shows typical changes of PRES in both occipital regions, and MRA (time of flight sequence) revealed segmental narrowing in multiple cerebral arteries (RCVS)
Comments
This patient presented with typical history of TCH precipitated by unaccustomed exercise and intracranial bleeding had been excluded. In the present case, the MRA clearly showed multiple areas of segmental narrowing suggestive of vasoconstriction/spasms which included the trunk of the basilar artery as well. So, the underlying pathology here had been RCVS though reversibility had not been demonstrated. The MR imaging of brain revealed symmetrical areas of hyperintensities in T2 sequences in both occipital areas as also in the right parasaggital region. These changes were suggestive of PRES, a common sequelae of RCVS.
Having found such significant abnormalities both in the cerebral vasculature and in the brain parenchyma, we cannot diagnose her headache as a case of “primary” exercise induced headache. This should be classified as a secondary headache disorder and designated as acute headache attributed to RCVS The presenting symptom of thunderclap headache would also be classified as secondary.
Features associated with RCVS are TCH, evidence for vasoconstriction of the intracranial arteries that reverses without specific therapy within 3 months and normal or near-normal CSF analysis. RCVS is possibly related to transient dysregulation of cerebral vascular tonicity resulting in multifocal arterial constriction and dilatation; however, the exact pathophysiology is unknown [47••, 48,49,50,51, 52•].
The major differential is with primary angitis of the CNS (PACNS). Both conditions have overlapping clinical features and demographics. The clinical setting in which symptoms developed, especially triggers such as vasoconstrictive drugs or childbirth, are different—the former favoring PANCS and the latter RCVS. The onset of headache in RCVS is usually dramatic (TCH), with patients rushed to ER for urgent evaluation. In absence of aneurysmal rupture, recurrent TCH is virtually diagnostic for RCVS; recurrences do not occur in any other condition associated with TCH. PACNS onset is generally insidious, with dull headaches and progressive neurologic deficits. RCVS patients present with raised BP and headache and the BP generally settles down quickly. Focal neurologic deficits are more common in PACNS, and these include hemiparesis and dysphasia. Cortical visual symptoms are commoner in RCVS, due to the posterior location of the associated PRES. It has been opined that Balint’s is mostly caused by RCVS with PRES.
Patients with RCVS may have normal neuroimaging despite widespread vasoconstriction, or may reveal a spectrum of abnormalities. The picture may change from day to day. The abnormalities include infarcts, often multiple, at times hemispheric border zones, parenchymal hemorrhages, convexity subarachnoid hemorrhages and vasogenic oedema (PRES). A normal scan in a patient with acute headache becoming abnormal within a few days time is classical of RCVS. PANCS patients, on the other hand, present with lesions in the initial scans. Such lesions are usually infarcts, often involving deep structures in the brain and brain stem. The FLAIR “dot sign” is more common in RCVS and is associated with PRES, supporting the hypothesis of distal vasoconstriction–vasodilation as a common pathophysiologic mechanism. Finally, it appears that recurrent TCH, or the combination of single TCH with normal brain imaging or cortical-only infarctions or vasogenic edema, probably has a predictive value of 100% for the diagnosis of RCVS.
PRES has been reported in 8–38% of RCVS cases in several series. Conversely, RCVS vascular changes have been noted in cases of PRES. These reports suggest a causal link between the conditions. PRES has similar clinical features as RCVS with headache, blurred vision, and seizures in the setting of bilateral, reversible vasogenic edema predominantly in the posterior cortical regions on CT and MRI. The vasogenic edema usually reverses completely, but can be complicated by hemorrhage or ischemic stroke. These occur in similar watershed locations to strokes in RCVS. PRES is commonly associated with hypertension as is RCVS, although 20–30% of patients with PRES are normotensive. The overlap in clinical and imaging features of RCVS and PRES suggest the two conditions may represent a spectrum of the same pathophysiological process
Case 9
A 74-year-old retired physician was admitted with a 5 days history of recent onset of acute confusion along with occasional comprehension of speech problem and lapses in recent memory. He also had difficulty in wearing clothes in the right way and developed difficulty in reading the newspaper. He became incontinent. He was normotensive and euglycemic. He was maintaining all activities of daily living reasonably well prior to the present illness.
Examination showed a disoriented elderly man with some restlessness and cognitive decline in the form of impaired recent memory, comprehensive speech defect, inability to write or read along with difficulty in putting his shirt on. Visual acuity was difficult to assess because of his confusion. There was no paresis of limbs. Muscle stretch reflexes were symmetrically brisk. He had bilateral Babinski signs. There were no meningeal signs. Routine hematological and biochemical investigations including thyroid function tests, thyroid autoantibody profile, and serum ammonia levels were all within normal limits. Connective tissue disease markers were all negative. CSF was normal except for a small rise in the protein content at 75 mg%. Chest X-ray was normal. Brain MRI showed bilateral symmetrical hyperintensities in the white matter of the parieto-occipital regions on T2-weighted FLAIR images. Several dots of hypointensity were detected in the hyperintense regions suggestive of microbleeds (Fig. 13). Restricted diffusion with hyperintensity was noted in DW1 images. In view of the patient being normotensive and the restricted diffusion noted in the DW1 images, we favored a diagnosis of hemorrhagic ADEM over PRES with microbleeds. The patient was given a single dose of methylprednisolone intravenously by infusion, and within 12 h, his confusion improved. Four more doses of methylprednisolone were administered over the next 4 days followed by a tapering course of oral steroids. The patient became fully alert and oriented. A repeat brain MRI after 3 months showed complete resolution of the lesions in the parieto-occipital lobes (Fig. 14).
The patient remained well for the next 8 months when he again developed intermittent disorientation and short-term memory lapses. He was re-investigated. While hematological and biochemical parameters remained normal, MRI of the brain again revealed bilateral hyperintensities mostly in the posterior regions of both cerebral hemispheres (Fig. 15).
This time he was treated with oral methylprednisolone 48 mg daily for the first week after which the dosage was gradually tailed off. He became normal by 1 week and had remained so when last evaluated 4 months later except for occasional problems with short-term memory.
Comments
The present case highlights an important diagnostic puzzle—differentiating PRES from hemorrhagic ADEM as microbleeds may occur in both conditions. At the initial stage of management, absence of hypertension favored the diagnosis of ADEM rather than PRES. Furthermore, the presence of diffusion restriction in DW1 sequences had also been a pointer in favor of ADEM rather than PRES. However, diffusion may be restricted in a small percentage of patients with PRES as well [53••]. Lastly, the rather dramatic improvement in the clinical status of the patient with resolution of the lesions on MR imaging, following use of parenteral corticosteroids favored the diagnosis of a demyelinating disorder. In general, corticosteroids are contraindicated in PRES as they may lead to clinical deterioration [53•]. However, rarely corticosteroids may enhance recovery in some patients with PRES when usual supportive measures fail to produce any improvement in clinical status [54,55,56].
This patient had a relapse of his disease 8 months later with re-appearance of similar MRI features. Clinical benefit was obtained with use of oral corticosteroids. It is well known that ADEM is not always a monophasic illness and both recurrent and relapsing forms of the disease have been described [57••]. In the present case, with a gap of eight months between the two episodes, a diagnosis of recurrent ADEM was entertained. On rare occasions, PRES may be recurrent as well if the primary causative disease continues to persist [58•, 59]. In a cohort study of eighteen children with renal disease, two patients each had three recurrent hypertensive episodes triggering PRES, with the earliest recurrence as early as 5 months after the initial episode [60•, 61]. Recurrent PRES may be triggered by hypertension, inflammation or endothelial injury [60•]. The present case experienced recurrence of PRES like MRI changes 8 months after hospital discharge which is similar to other cases previously described [30]. There is debate on the true reversibility of PRES, as complications including intracranial hemorrhage and permanent neurologic sequelae have been reported [62••, 63•, 64].
General Comments
PRES is now a well-recognized neurological disorder. It is characterized predominantly by white matter edema affecting the occipital and posterior parietal lobes of the brain. In most cases, the pathology is reversible if properly treated. The fundamental pathophysiological mechanism of PRES in most cases is dysregulation of the autoregulatory mechanism of the cerebral circulation. One hypothesis is that high blood pressure causes cerebrovascular autoregulation failure, resulting in hyperperfusion. Another is that hypoperfusion is caused by dysregulated vasospasm developing as a response to high blood pressure to maintain constancy of cerebral perfusion.
In either case, there is endothelial damage at the BBB. Some toxic inflammatory cytokines may cause BBB damage indirectly by altering the luminal diameter of feeding arterioles. Hypoperfusion may also result from clogging of the microvasculature by parasitized red blood cells in cerebral malaria producing the same effect [65••].
The characteristic neuroimaging abnormality in PRES is the presence of white matter signal changes localized to the parieto-occipital regions of the brain. White matter signal changes are usually symmetric and tend to spare the calcarine and paramedian parts of the occipital lobes and often do not extend beyond the sylvian fissure. Many atypical structures such as the frontal lobes, temporal lobes, basal ganglia, cerebellum, brainstem, and thalamus may also be involved, but in lesser frequency [8••]. Importantly, PRES needs differentiation from cerebral venous sinus thrombosis, posterior circulation strokes specially basilar artery thrombosis, primary vasculitis of CNS and autoimmune encephalitis.
Concluding Remarks
PRES is a major manifestation in patients who are hypertensive, have renal disease, post-partum, or have a sepsis syndrome. Hemorrhages in areas of vasogenic oedema occur in 10–20% of cases, and PRES should be suspected in patients with a sudden surge of hypertension, poor kidney function, autoimmune disease, and evolving gram-positive sepsis. The pathogenesis is unclear. Both occurrence of vasogenic and cytotoxic oedema had been proposed. A closer look at the cases cited in this communication would suggest that in most both factors tend to play a part. The fundamental problem is one of cerebrovascular circulatory dysregulation with vasodilatation causing excess exudation of water and plasma out of the arterioles causing vasogenic oedema and vasoconstriction causing tissue hypoperfusion inducing cytotoxic oedema. Cytotoxic edema may also result from inflammatory states through liberation of toxins and cytokines and hypoperfusion as in sepsis with encephalopathy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Truwit CL, Denaro CP, Lake JR, DeMarco T. MR imaging of reversible cyclosporin A-induced neurotoxicity. Am J Neuroradiol. 1991;12:651–9.
Schwartz RB, Bravo SM, Klufas RA, et al. Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. Am J Roentgenol. 1995;165:627–31.
Hauser RA, Lacey DM, Knight MR. Hypertensive encephalopathy. Magnetic resonance imaging demonstration of reversible cortical and white matter lesions. Arch Neurol. 1988;45:1078–83.
Rail DL, Perkin GD. Computerized tomographic appearance of hypertensive encephalopathy. Arch Neurol. 1980;37:310–1.
Jones-Muhammad M, Warrington JP. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci. 2019;9:224–39.
Hobson EV, Craven I, Blank SC. Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness. Perit Dial Int. 2012;32(6):590–4.
•• Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. https://doi.org/10.1056/NEJM199602223340803. One of the first articles to describe the syndrome of PRES and its pathophysiology. Reversible, predominantly posterior leukoencephalopathy may develop in patients who have renal insufficiency or hypertension or who are immunosuppressed. The findings on neuroimaging are characteristic of subcortical edema without infarction
•• Raman R, Devaramane R, Jagadish GM, Chowdaiah S. Various imaging manifestations of posterior reversible encephalopathy syndrome (PRES) on magnetic resonance imaging (MRI). Pol J Radiol. 2017;82:64–70. https://doi.org/10.12659/PJR.899960. A detailed radiological study. The involvement of the parieto-occipital, frontal and temporal lobes is common in PRES. Occasionally, there may be an involvement of the basal ganglia, cerebellum and brainstem, with or without hemorrhage and restricted diffusion. Radiologists should be aware of the typical and atypical imaging manifestations of PRES in order to make an accurate diagnosis
•• Je F, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–25. A diagnosis of PRES should be considered in the setting of acute neurological symptoms in patients with renal failure, blood pressure fluctuations, use of cytotoxic drugs, autoimmune disorders, or eclampsia. Characteristic radiographic findings include bilateral regions of subcortical vasogenic oedema that resolve within days or weeks. The presence of haemorrhage, restricted diffusion, contrast enhancement, and vasoconstriction are all compatible with a diagnosis. In most cases, PRES resolves spontaneously and patients show both clinical and radiological improvements. The range of symptoms that can comprise the syndrome might be broader than usually thought
•• Anderson RC, Patel V, Sheikh-Bahaei N, CSJ L, Rajamohan AG, Shiroishi MS, Kim PE, Go JL, Lerner A, Acharya J. Posterior reversible encephalopathy syndrome (PRES): pathophysiology and neuro-imaging. Front Neurol. 2020;11:463. https://doi.org/10.3389/fneur.2020.00463. Posterior reversible encephalopathy syndrome (PRES) represents a unique clinical entity with non-specific clinical symptoms and unique neuroradiological findings. This syndrome may present with a broad range of clinical symptoms from headache and visual disturbances to seizure and altered mentation. Typical imaging findings include posterior-circulation predominant vasogenic edema. Although there are many well-documented diseases associated with PRES, the exact pathophysiologic mechanism has yet to be fully elucidated. Generally accepted theories revolve around disruption of the blood-brain barrier secondary to elevated intracranial pressures or endothelial injury
Garg RK, Kumar N, Malhotra HS. Posterior reversible encephalopathy syndrome in eclampsia. Neurol India. 2018;66:1316–23.
Jones-Muhammad M, Warrington JP. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci. 2019;9:224–39.
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80.
•• Chakravarty A, Chakrabarti SD. The neurology of eclampsia: some observations. Neurol India. 2002;50:128–35. An early report on the neurological and radiological findings in eclampsia and a detailed discussion on the pathogenesis of the condition
Qureshi M, Huang J. Atypical case of posterior reversible encephalopathy in a pregnant patient without pre-eclampsia. Cureus. 2019;11(9):e5620. https://doi.org/10.7759/cureus.5620.
• Delgado ME, Del Brutto OH. Case report: Reversible posterior leukoencephalopathy in a venomous snake (Bothrops asper) bite victim. Am J Trop Med Hyg. 2012;86(3):496–8. Unusual case report of PRES induced by snake bite
• Ibrahim AM, TT ES, Ghanem M, Fayed AM, Shaban NA. A horned viper bite victim with PRES. Case Rep Neurol Med. 2017;2017:6. https://doi.org/10.1155/2017/1835796. Unusual case report of PRES induced by snake bite
Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am. J. Neuroradiol. 2008;29:1043–9.
Tam CS, Galanos J, Seymour JF, et al. Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies. Am J Hematol. 2004;77:72–6.
• Largeau B, Boels D, Victorri-Vigneau C, Cohen C, Gandonnière CS, Ehrman S. Posterior reversible encephalopathy syndrome in clinical toxicology: a systematic review of published case reports. Front Neurol. 2020; https://doi.org/10.3389/fneur.2019.01420. This study highlights that comorbidities such as chronic hypertension and kidney failure were less frequent than in patients with other PRES etiologies. Imaging analysis did not highlight a specific pattern for poisoning-induced PRES. Although less described, PRES in the context of poisoning, which shares most of the clinical and radiological characteristics of other etiologies, is not to be ignored.
Dhabhar J, Mehta V, Desai N. Optic neuritis after a snakebite: a diagnostic dilemma. Ochsner J. 2021;21(1):90–2. https://doi.org/10.31486/toj.19.0014.
Sengupta P, Biswas S. Dialysis disequilibrium leading to posterior reversible encephalopathy syndrome in chronic renal failure. CEN Case Rep. 2016;5(2):154–7. https://doi.org/10.1007/s13730-016-0215-4.
Canney M, Kelly D, Clarkson M. Posterior reversible encephalopathy syndrome in end-stage kidney disease: not strictly posterior or reversible. Am J Nephrol. 2015;41:177–82. https://doi.org/10.1159/000381316.
•• Magro-Checa C, Steup-Beekman GM, Huizinga TW TW, van Buchem MA, Ronen I. Laboratory and neuroimaging biomarkers in neuropsychiatric systemic lupus erythematosus: where do we stand, Where To Go? Front Med. 2018;5:340. This article critically reviews the current state of knowledge on laboratory and neuroimaging biomarkers in NP-SLE, discusses the factors that need to be addressed to make these biomarkers suitable for clinical application, and suggests potential future research paths to address important unmet needs in the NP-SLE field
Sommerlad A, Duncan J, Lunn MPT, Foong J. Neuropsychiatric systemic lupus erythematosus: a diagnostic challenge. BMJ Case Rep. 2015; https://doi.org/10.1136/bcr-2014-208215.
Hoffman RW, Maldonado ME. Immune pathogenesis of mixed connective tissue disease: a short analytical review. Clin Immunol. 2008;128(1):8–17. https://doi.org/10.1016/j.clim.2008.03.461.
Liu B, Zhang X, Zhang FC, Yao Y, Zhou R, Miao-Miao Xin MM, Wang LQ. Posterior reversible encephalopathy syndrome could be an underestimated variant of “reversible neurological deficits” in Systemic Lupus Erythematosus. BMC Neurol. 2012;12:152.
• Nisar T, Alchaki AR, Feinstein E. A rare case of cyclophosphamide-induced posterior reversible encephalopathy syndrome in a patient with anti-GBM vasculitis, and review of current literature. Case Rep Neurol Med. 2019;2019:5. https://doi.org/10.1155/2019/2418597. A case of cyclophosphamide-induced PRES in a patient with anti-glomerular basement membrane (Anti-GBM) positive vasculitis. In the acute setting, PRES can be challenging to distinguish from cerebral venous sinus thrombosis or cerebral vasculitis based on clinical presentation. Neuroimaging with magnetic resonance imaging (MRI) of the brain along with a vessel imaging, can help reach the diagnosis
Kim JM, Son CN, Chang HW, Kim SH. Simultaneous presentation of acute disseminated encephalomyelitis (ADEM) and systemic lupus erythematosus (SLE) after enteroviral infection: Can ADEM present as the first manifestation of SLE? Lupus. 2015;24:633–7. https://doi.org/10.1177/0961203314560426.
Pavicic Ivelja M, Dolic K, Marasovic Krstulovic D, Glavina G, Ivic I. Case of acute disseminated encephalomyelitis associated with cytomegalovirus reactivation in an immunocompromised systemic lupus erythematosus patient. Medicina (Kaunas). 2021;57(9):882. https://doi.org/10.3390/medicina57090882.
•• Mackay M, Tang CC, Vo A. Advanced neuroimaging in neuropsychiatric systemic lupus erythematosus. Curr Opin Neurol. 2020;33(3):353–61. https://doi.org/10.1097/WCO.0000000000000822. Study design issues related to patient selection (non-NPSLE vs. NPSLE syndromes, SLE disease activity, medications) are critical for biomarker development. Regional and network structural and functional changes identified with advanced brain imaging techniques in patients with non-NPSLE may be further developed as biomarkers for cognitive and mood disorders attributable to SLE-related mechanisms
Patel UV, Patel NJ. Posterior reversible leukoencephalopathy syndrome as a presenting manifestation of p-ANCA-associated vasculitis. BMJ Case Rep. 2014; https://doi.org/10.1136/bcr-2013-202022.
Barber CE, et al. Posterior reversible encephalopathy syndrome: an emerging disease manifestation in systemic lupus erythematosus. Semin Arthritis Rheum. 2011;41:353–63.
• Sheikh-Bahaei N, Acharya J, Rajamohan A, Kim PE. Advanced imaging techniques in diagnosis of posterior reversible encephalopathy syndrome (PRES). Front Neurol. 2020;11:165. https://doi.org/10.3389/fneur.2020.00165. Advanced imaging techniques, such as MR spectroscopy or positron emission tomography (PET) can provide additional information to determine the diagnosis. Other techniques, such as susceptibility weighted imaging (SWI) improves detection of hemorrhage which has prognostic role. CT or MR Perfusion as well as Single-Photon Emission Computed Tomography (SPECT) are more useful to understand the underlying vasculopathic changes in PRES and may answer some of the unresolved controversies in pathophysiology of this complex disease.
Kuhn JH, Peters CJ. Arthropod-borne and rodent-borne virus infection. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal Medicine, vol. 2. 19th ed. New York: McGraw-Hill; 2015. p. 1318–9.
Sawant Y, Birajdar S, Doshi H, et al. posterior reversible encephalopathy (PRES) in a child with severe dengue. J Trop Pediatr. 2020;66(3):322–6.
•• Trivedi S, Chakravarty A. Neurological complications of dengue fever. Curr Neurol Neurosci Rep. 2022;22(8):515–29. An uptodate review of the neurological manifestations of Dengue fever including their pathogenesis
Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Microvascular and endothelial function for risk prediction in dengue: an observational study. Lancet. 2015;385(Suppl 1):S102. https://doi.org/10.1016/S0140-6736(15)60417-2.
Sohoni CA. Bilateral symmetrical parieto occipital involvement in dengue infection. Ann Indian Acad Neurol. 2015;18:358–9. https://doi.org/10.4103/0972-2327.160096.
Wang WH, Urbina AN, Chang MR, Assavalapsakul W, Lu PL, Chen YH, Wang SF. Dengue hemorrhagic fever: a systematic literature review of current perspectives on pathogenesis, prevention and control. J Microbiol Immunol Infect. 2020; https://doi.org/10.1016/j.jmii.2020.03.007.
Mai NTH, Phu NH, Nghia HDT, et al. Dengue-associated posterior reversible encephalopathy syndrome. Vietnam. Emerg Infect Dis. 2018;24(2):402–4.
•• Cudalbu C, Taylor-Robinson SD. Brain edema in chronic hepatic encephalopathy. J Clin Exp Hepatol. 2019;9(3):362–82. This review explores the different methods used to measure brain edema ex vivo and in vivo in animal models and in humans with chronic HE. In addition, an in-depth description of the main studies performed to date is provided. The role of brain edema in the neurological alterations linked to HE and whether HE and brain edema are the manifestations of the same pathophysiological mechanism or two different cerebral manifestations of brain dysfunction in liver disease are still under debate. In vivo MRI/magnetic resonance spectroscopy studies have allowed insight into the development of brain edema in chronic HE
Alhilali LM, Reynolds AR, Fakhran S. A multi-disciplinary model of risk factors for fatal outcome in posterior reversible encephalopathy syndrome. J Neurol Sci. 2014;347:59–65. https://doi.org/10.1016/j.jns.2014.09.019.
Chawla R, Smith D, Marik PE. Near fatal posterior reversible encephalopathy syndrome complicating chronic liver failure and treated by induced hypothermia and dialysis: a case report. J Med Case Rep. 2009;26(3):6623. https://doi.org/10.1186/1752-1947-3-6623.
Elizabeth J, Ramy S, Ishita D, Kristina K, Sharma R. PRES in alcoholic hepatitis: hepatic encephalopathy a common theme: 1778. Am J Gastroenterol. 2016;111:S852–3.
•• Kumar G, Taneja A, Kandiah PA. Brain and the liver: cerebral edema, hepatic encephalopathy and beyond. Hepatic Critical Care. 2017:83–103. https://doi.org/10.1007/978-3-319-66432-3_8. Occurrence of brain dysfunction is common in both chronic liver disease as well as acute liver failure. While brain dysfunction most commonly manifests as hepatic encephalopathy is chronic liver disease; devastating complications of cerebral edema and brain herniation syndromes may occur with acute liver failure. Ammonia seems to play a central role in the pathogenesis of brain dysfunction in both chronic liver disease and acute liver failure. In this chapter we outline the pathophysiology and clinical management of brain dysfunction in the critically ill patients with liver disease
•• de Boysson H, , Parienti JJ, Mawet J, et al Primary angiitis of the CNS and reversible cerebral vasoconstriction syndrome: a comparative study. Neurology 2018;91:e1468-e1478.This study confirms that careful analysis of clinical context, headache features, and patterns of brain lesions can distinguish PACNS and RCVS within the first few days of admission in most cases. However, diagnosis remains challenging in a few cases.
•• Singhal AB, Topcuoglu FJW, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol. 2016;79:882–94. Recurrent thunderclap headache (TCH), and single TCH combined with either normal neuroimaging, border zone infarcts, or vasogenic edema, have 100% positive predictive value for diagnosing RCVS or RCVS-spectrum disorders. In patients without TCH and positive angiography, neuroimaging can discriminate RCVS (no lesion) from PACNS (deep/brainstem infarcts).
Choi HA, Lee MJ, Choi H, Chung CS. Characteristics and demographics of reversible cerebral vasoconstriction syndrome: a large prospective series of Korean patients. Cephalalgia. 2018;38(4):765–75.
Singhal AB. Diagnostic challenges in RCVS, PACNS, and other cerebral arteriopathies. Cephalalgia. 2011;31(10):1067–70.
•• Singhal AB. Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome as syndromes of cerebrovascular dysregulation. Continuum (Minneap Minn). 2021;27(5, Neurocritical Care):1301–20. This article describes the causes, clinical and imaging features, management, and prognosis of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS), in which the underlying pathophysiology is related to reversible dysregulation of the cerebral vasculature.
• Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke. 2012;43(3):860–2. Preliminary results suggest that high-resolution contrast-enhanced vessel wall MRI may enable differentiation between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis
• Wagih A, Mohsen L, Rayan MM, Hasan MM, Al-Sherif AH. Posterior reversible encephalopathy syndrome (PRES): restricted diffusion does not necessarily mean irreversibility. Pol J Radiol. 2015;80:210–6. https://doi.org/10.12659/PJR.893460. PRES is completely reversible in the majority of patients, even with restricted diffusion. None of the variables under study could predict the reversibility of PRES lesions. It seems that this process is individual-dependent
Parikh NS, Schweitzer AD, Young RJ, Giambrone AE, Lyo J, Karimi S, Knobel A, Gupta A, Navi BB. Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. J Neurol Sci. 2017;15(380):11–5. https://doi.org/10.1016/j.jns.2017.06.044.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13.
Irvin W, MacDonald G, Smith JK, Kim WY. Dexamethasone- induced posterior reversible encephalopathy syndrome. J Clin Oncol. 2007;25:2484–6.
•• Kariyawasam S, Singh RR, Gadian J, Lumsden DE, Lin J-P, Siddiqui A, Hacohen Y, Absoud M, Lim M. Clinical and radiological features of recurrent demyelination following acute disseminated encephalomyelitis (ADEM), multiple sclerosis and related disorders. Mult Scler Relat Disord. 2015;4(5):451–6. https://doi.org/10.1016/j.msard.2015.06.013. ADEM patients with infratentorial demyelination are more likely to present with a second infratentorial demyelination, although clinical and radiological features at outset could not predict the relapsing cohort
• Ergün T, Lakadamyali H, Yilmaz A. Recurrent posterior reversible encephalopathy syndrome in a hypertensive patient with end-stage renal disease. Diagn Interv Radiol. 2008;14(4):182–5. Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiologic entity characterized by headache, variable mental status, epilepsy, visual disturbances, and typical transient changes in the posterior cerebral perfusion. Recurrence of PRES is not common, but increasingly in recent years, studies demonstrate recurrence of this syndrome in populations with different diseases. In this report, we describe recurrent PRES in a hypertensive patient with end-stage renal disease, and discuss recurrence as the least-characterized feature of PRES. This condition can cause neurological sequelae such as persistent brain damage and epilepsy, arising from delays in diagnosis and therapy. To the best of our knowledge, this is the first report demonstrating recurrent PRES in a patient on hemodialysis for end-stage renal disease
Pande AR, Ando K, Ishikura R, et al. Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med. 2006;24:659–68.
• Prasad N, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol. 2003;18:1161–6. Leukoencephalopathy with severe hypertension is reversible both clinically and radiologically in the majority of children after the control of hypertension. However, a few patients may have residual damage and may need psychometric analysis and follow-up for neurodevelopmental sequelae
Cordelli DM, Masetti R, Ricci E, Toni F, Zama D, Maffei M, Gentili A, Parmeggiani A, Pession A, Franzoni E. Life-threatening complications of posterior reversible encephalopathy syndrome in children. Eur J Paediatr Neurol. 2014;18(5):632–40.
•• Hinduja A. Posterior reversible encephalopathy syndrome: clinical features and outcome. Front Neurol. 2020;11:71. https://doi.org/10.3389/fneur.2020.00071. Posterior reversible encephalopathy syndrome (PRES) is an acute neurotoxic syndrome that is characterized by a spectrum neurological and radiological feature from various risk factors. While clinical and radiographic reversibility is common, long-standing morbidity and mortality can occur in severe forms. In patients with malignant forms of PRES, aggressive care has markedly reduced mortality and improved functional outcomes. Although seizures were common, epilepsy is rare. Various factors that have been associated with poor outcome include altered sensorium, hypertensive etiology, hyperglycemia, longer time to control the causative factor, elevated C reactive protein, coagulopathy, extensive cerebral edema, and hemorrhage on imaging
• Sweany JM, Bartynski WS, Boardman JF. “Recurrent” posterior reversible encephalopathy syndrome. J Comput Assist Tomogr. 2007;31(1):148–56. In a retrospective review, 3 (3.8%) of 78 patients developed recurrent posterior reversible encephalopathy syndrome. Underlying clinical conditions included sickle cell disease, antibody-positive autoimmune disease, and allogeneic bone marrow transplantation. Infection (bacterial/viral) was suspected or documented in both episodes in all 3 patients. Evidence of endothelial injury (schistocyte formation and increased lactate dehydrogenase) was documented in all patients, and multiple organ dysfunction syndrome developed during the hospital course of all admissions
Prasad N, Gulat S, Gupta RK, Kumar Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol. 18(11):1161–6.
•• Trivedi S, Chakravarty A. Neurological complications of malaria. Curr Neurol Neurosci Rep. 2022;22(8):499–513. A up to date review of neurological manifestations of Malaria including thir pathophysiology
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Jasodhara Chaudhuri, Sagar Basu, Mrinal K Roy, and Ambar Chakravarty each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhuri, J., Basu, S., Roy, M.K. et al. Posterior Reversible Leucoencephalopathy Syndrome: Case Series, Comments, and Diagnostic Dilemma. Curr Neurol Neurosci Rep 23, 433–449 (2023). https://doi.org/10.1007/s11910-023-01281-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01281-3