Abstract
Introduction
Most women with alloimmune cause of recurrent spontaneous abortion (RSA) includes increased sharing of human leukocyte antigens (HLA) that may prohibit the mother from making anti-paternal cyto-toxic antibodies (APCA), anti-idiotypic antibodies (Ab2) and mixed lymphocyte reaction blocking antibodies (MLR-Bf). Overactivity of T helper-1 (Th-1) cytokines and natural killer (NK) cells have been also reported to be the major alloimmune cause of recurrent spontaneous abortion (RSA). It was revealed from extensive updated analysis of this subject that paternal lymphocytes immunotherapy may play a significant role in the prevention of alloimmune cause of fetal loss in women with RSA. These alloimmune parameters are found to be suppressed in successful immunotherapy, which is comparable to normal pregnancy.
Review and discussion
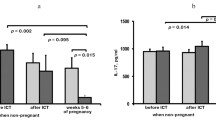
Various studies represented that paternal lymphocyte immunotherapy was attributed to the high expression of APCA, Ab2, MLR-Bf and inhibition of Th-1 pattern of cytokines and NK cell activity in women with alloimmune cause of RSA. Present updated randomized clinical trials demonstrated that women with RSA of study group who have been treated with paternal lymphocyte immunotherapy had more successful outcomes (68%) as compared to women with RSA of control group who either received autologous lymphocytes/third party lymphocytes/normal saline or no therapy (54%), (p<0.02). However, when the results of the randomized and nonrandomized studies were pooled together it was observed that 67% of women with RSA of study group who received paternal lymphocyte immunotherapy showed successful pregnancy outcome in comparison to 36% success in women with RSA of control group who either received autologous lymphocytes/third party lymphocytes/normal saline or no therapy (p<0.05).
Conclusion
These results advocate the role of paternal lymphocyte immunotherapy for the maintenance of pregnancy in women with RSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been reported that recurrent miscarriage is the commonest complication of pregnancy affecting approximately 1 in 300 pregnant women [100, 118]. Recurrent spontaneous abortion (RSA) can be defined as occurrence of three or more clinically detectable pregnancy failure before the 20th weeks of gestation from the last menstrual period or less than 500 g of fetal body weight [21, 29, 105]. In the vast majority of the cases, the etiology is unknown and several hypotheses have been proposed on the basis of available data. These have varied from genetic [78, 103], anatomical [43, 102, 120], endocrine [19], placental anomalies [49, 62, 116], smoking and alcohol consumption [37], exposure to environmental factors such as lead, mercury, ethylene oxide and ionizing radiations [89], and to immunological factors [1, 2, 3, 48, 56, 67, 79, 83, 87, 92, 112]. RSA can be classified into primary RSA aborters and secondary RSA aborters. Primary RSA aborters are those who have lost all previous pregnancies and have no live birth. Secondary RSA aborters are those who have at least one successful pregnancy irrespective of the number of pregnancy losses. Epidemiological studies suggest that the risk of subsequent pregnancy loss is approximately 24% after two clinical pregnancy losses, 30% after three and 40% after four consecutive spontaneous abortions [95, 117].
The immunological relationship between the mother and the fetus is a bidirectional communication determined on one hand by fetal antigen presentation and on other hand by recognition and reaction to these antigens by the maternal immune system. There are evidences that reveal that immunological recognition of pregnancy is important for the maintenance of gestation and inadequate recognition of fetal antigens might lead to abortion in women with RSA [22, 93]. Alloimmunity has been indicated in several studies by showing an association of habitual abortion with an increased sharing of human leukocyte antigens (HLA) with the father that may prohibit the production of anti-paternal cyto-toxic antibodies (APCA), anti-idiotypic antibodies (Ab2) and mixed lymphocyte reaction blocking antibodies (MLR-Bf). Immunotherapy with paternal lymphocytes is an effective treatment for unexplained recurrent spontaneous abortions as it was attributed to the production of APCA [1, 65, 75, 79, 96], Ab2 [48, 65, 80, 106] and MLR-Bf [1, 2, 3, 9, 26, 63, 68, 75, 83, 90, 93, 100, 110, 111, 112, 125] during pregnancy in women with RSA. These antibodies may play an important role in the maintenance of pregnancy. Where as several studies have shown that the absence or low expression of APCA, Ab2 and MLR-Bf may cause recurrent fetal loss in women with RSA [2, 3, 65, 68, 80, 83, 93].
Several studies have reported that activation of the maternal NK cells induces subsequent abortion in women with normal chromosomes. The proposed mechanism is that maternal NK cells activate Th1 cells for secretion of cytokines that are toxic to the trophoblast [17, 27, 55, 93, 122]. Hence it is possible that suppression of NK cell activity may help in the maintenance of the fetal allograft during pregnancy. However, decidual NK cells are not cytolytic, but produce IFNγ which activates the decidual macrophage (MØ) for the production of high levels of nitric oxide (NO) and tumor necrosis factor-alfa (TNFα) which damage to the conceptus not by direct lysis of trophoblast cells but through apoptosis and causes the inhibition of secretion of granulocyte macrophage colony stimulating factors (GM-CSF) from the uterine epithelium [35, 119, 121, 123]. MØ also produces IL-12 in response to certain microbial products (LPS, IFNγ) during infectious diseases, which stimulates NK cells and cytotoxic T cells (CTLs) to secrete IFNγ that enhances Th1 development and induces the secretion of tumor necrosis factor-alfa (TNF-α), TNF-beta (TNF-β) and interleukin-2 (IL-2). These Th-1 cytokines may proposed to stimulate pregnancy failure via embryo and trophoblast toxicity [5, 18, 42, 43, 45, 64, 88, 91, 92]. Whereas Th2 cells produce IL-4, IL-5, IL-10 and IL-13 which promote success of the pregnancy by counter inflammation and suppression of the NK cell activity [17, 18, 20, 36, 38, 40, 43, 44, 59, 91, 98, 99]. The predominance of a given cytokines in the microenvironment at the time of antigen presentation is an important factor in driving naive CD4+T cells toward Th-1 or Th-2 dominated population. In some of the recent studies it has been demonstrated that paternal lymphocyte immunotherapy caused significant inhibition in the level of Th-1 cytokines and NK cells activity [32, 39, 61].
Mechanism of paternal lymphocyte immunotherapy in women with RSA
The exact mechanisms of immunotherapy against paternal lymphocytes have yet to be elucidated. The initial reports on paternal lymphocyte immunization used the sharing of HLA antigens between spouses as a criteria for immunization [9, 115]. This excess sharing was considered to be increased in couples with RSA [56] and responsible for the hyporesponsiveness was considered to be shown by a lower incidence of APCA, Ab2 and MLR-Bf in RSA couples [75]. Some investigators suggested that paternal lymphocyte immunotherapy may act as immunogen to enhance the maternal immune response and to induce various humoral antibodies as an immunological regulators for maintaining pregnancy. It was further suggested that humoral antibodies (APCA, Ab2 and MLR-Bf) produced as a result of immunotherapy [83] would mask the fetal HLA antigens and prevent them from being attacked by the maternal T cells (Fig. 1). We have recently demonstrated that MLR-Bf developed as a result of lymphocyte immunotherapy was IgG3 in nature and associated with success of pregnancy in women with RSA.
Role of MLR-Bf in the maintenance of pregnancy. Trophoblast bears the P60 receptor and R80 K (80 kDa) antigens on its surface. P60 receptors when binds with the γδ T cell receptor of CD4 cells and R80 K antigen when recognized by the γδ T cell receptor of NK cells , Th1 patterns of cytokines (IFN γ, TNFα, and IL-2) are produced which kill the fetal cells). On the contrary αβ T cell receptor of CD8+ cells and P60 T cell receptor of trophoblast signals for secretion of Th2 pattern of cytokines (IL-4, IL-5, IL-10, IL-13) and MLR-Bf that inhibits NK activity and the activity of TNF α producing macrophages and protects the fetal cells by lysis caused by host effector cells
The anti T cell receptor (TCR) idiotypic antibody that has been reported to be present in the sera of normal pregnant women [48] would provide another mechanism for the immunotherapy. After immunization with paternal lymphocytes, maternal T cells recognizing paternal HLA antigens (one of the HLA antigens of the fetus) would expand and serve as immunogens to produce anti TCR-idiotypic antibodies. The anti TCR idiotypic antibody would then bind specifically to the TCR and suppress the maternal immune response against the fetus, allowing the fetus to escape the maternal immunological attack. Thus the beneficial effect of this procedure has been attributed to the induction of various humoral antibodies that may block the mechanism of immune-rejection of the fetus and help in the implantation and fetal growth [48, 63, 68, 79, 90, 93, 125]. The beneficial effect of paternal lymphocyte immunotherapy also include specific and non specific T cell suppression [10, 71], a decrease in the level of maternal IL-2 receptors [53], which shifts Th1 to Th2 type immunity [39] and causes decrease in NK cell activity [61]. Various recent Th1/Th2 theories may support these beneficial effects to maintain pregnancy [40, 44, 64, 91, 92].
Recently Gafter et al. [32] demonstrated the culture of monocytes of alloimmunized women with RSA and found that paternal lymphocyte immunotherapy causes the reduction in level of IL-6. There were no previous reports about the serum levels of this pleiotropic cytokine and its receptor in women with RSA. The result obtained through this work showed that there are highly significant difference in the IL-6 and its soluble receptor levels between the women with RSA that had received and those who had not received lymphocyte immunotherapy. Some of the in vitro studies demonstrated that low dose of IL-6 stimulate the asymmetric antibodies synthesis and a high dose decreases it. Thus it would be suggested that IL-6 regulate the synthesis of asymmetric antibodies in the trophoblast that block the placental antigen (R80K) [124] and NK cells [50]. HLA-G antigens expressed on trophoblast cells are reported to inhibit NK cell activity [30, 58, 69, 84, 97] during normal pregnancy. However, increased number of NK cells has been observed in women with RSA [6]. Embryo rejection in animal models also appears to depend upon activated natural killer (NK) cells rather than on antigen specific effector cells. Alternatively these proinflammatory cytokines may convert NK cells to lymphokine activated killer (LAK) cells, which have been shown to lyse trophoblast cells. High systemic levels of LAK cells may correlates with the high abortion rates. Paternal lymphocyte immunotherapy seems to develop anti-R80K asymmetric antibodies, which suppress the activity of NK cell and prevent the fetal loss in women with RSA.
It was further reported that trophoblast bears P60 receptor and R80 K (80 kDa) antigens on its surface. P60 receptors when binds to the gamma delta T (γδ T) cell receptor of CD4 cells and R80 K antigen gets recognized by the γδ T cell receptor of NK cells, Th1 patterns of cytokines are produced which kill the fetal cells [5, 119]. NK-γδ T cells and γδ T cells have been implicated in fetal resorptions and elimination of these NK-T cells prevents abortions. On the contrary, when alpha beta-T (αβ-T) cell receptor of CD8+ cells and P60 T cell receptor of trophoblast cells bind with each other, CD8+ cells may become CD4– CD8–. This modified CD8+cells has anti-inflammatory properties and cause the change in the signal for secretion of Th2 pattern of cytokines (Fig. 1). Arck et al. [5] points out that these populations have different and opposite effects when activated, the NK γδ T cell is more Th1-like as they produce IFNγ, TNFα and IL-2, while the γδ T cell is more Th2-like as it secretes IL-10 and TGFβ2.
It has been explained that the failure of anti CD4 and anti CD8 antibodies decrease the abortion rates in mice with recurrent spontaneous abortion [23, 31]. Injection of anti γδ TCR antibodies, anti-IFNγ antibodies and anti-NK antibodies into pregnant mice may decrease abortion rates. Lymphocyte immunization in women with RSA seems to cause transformation of CD8+ αβ cells to CD4– CD 8– cells as well as independent production of IL-4 and IL-10, which protects the abortion by inhibition of NK cell activation, counter inflammation and production of alloantibody formation [44, 59, 99]. Check et al. [15] was reported that lymphocyte immunization causes an increase in progesterone induced blocking factor (PIBF) in women with RSA, which may play a significant role in the maintenance of pregnancy by regulating the Th2 shift. This shift induces some T cells that utilize different TCR repertoires and possibly suppresses maternal rejection reactions and causes the maintenance and continuation of successful pregnancies [38, 39, 107]. Same effect was also predicted when paternal lymphocytes immunization induced APCA, Ab2 and MLR-Bf in women with RSA. An increased level of peripheral blood CD 56+ CD3− NK cell levels and NK cell cytotoxicity against K562 [41, 60] cells and significant decrease in these parameters [61, 108] has been noticed in alloimmunized women with RSA. In most women who experience recurrent miscarriage and no cause can be identified, the humoral and cellular immune mechanisms may be responsible which prevent mothers from developing protective immune responses which are essential for the survival of the semiallogeneic pregnancy. However, failure of these immune mechanisms has been proposed as a cause for 50% of all these losses. Thus the mechanism of this therapeutic effect in alloimmune pregnancy loss concerns with the expression of APCA, Ab2 and MLR-Bf as well as inhibition in the induction of Th1 cytokine and NK cell activity during pregnancy in women with RSA.
Various clinical trials of lymphocyte immunotherapy using for women with RSA
Taylor and Faulk [115] first reported paternal lymphocyte immunotherapy for women with unexplained RSA. Since then it has become widely performed with various reports of its efficacy and safety. They originally based their idea of third party lymphocyte immunization for women with RSA on observations that renal allograft rejection could be delayed by third party blood transfusions while Beer et al. [9] based paternal lymphocyte immunization on their belief that maternal “blocking antibodies” were necessary for successful pregnancy. Various recent studies demonstrated that humoral antibodies like APCA [60, 72, 79, 108] anti TCR idiotypic antibodies [48] and MLR-Bf [2, 3, 68, 83, 93] were produced during pregnancy as well as after paternal lymphocyte immunotherapy in women with RSA were correlated with the success of pregnancy. In addition, other beneficial effect of this therapeutic approach was found to be associated with non specific T cell suppression [10, 71], a decrease in the level of maternal IL-2 receptors [53], shift to Th2 type immunity [32, 39] and suppression of NK cell activity [32, 61]. Thus there is popular current belief that whenever there is failure of induction of these protective immune response, failure of pregnancy occurs [16]. Some of the published results of randomized allogenic lymphocyte immunotherapy for women with RSA did not confirm the efficacy of immunotherapy [14, 28, 47, 51, 63, 77]. They suggested that there are some other factors such as the number of previous miscarriages, presence of prior live births, and time of conception after immunization and patient’s age which may also influence the outcome of pregnancy [14]. However, a recent study [77] reported that this mode of therapeutic approach does not improve pregnancy outcome in women with RSA. But they did not analyze the results in the light of previous miscarriage numbers. They used paternal lymphocytes, which were stored for overnight rather than fresh cells, and did not fully excluded patients with autoimmunity. Stored cells might lose immunogenic effects and certain types of patients with autoimmunity, such as antiphospholipid antibody and antinuclear antibody, may not respond well. They considered the cases that did not achieve pregnancy in 12 months after immunotherapy, as a failure. However, our study and most of the other studies analyzed only the successful outcome. The data of immunotherapy during early pregnancy should be analyzed in the light of number of previous miscarriage and gestational window. Most of the controversial studies performed immunotherapy only once or twice before pregnancy whereas in most of the successful therapies immunization was performed at the regular interval of 2–4 weeks before the pregnancy and again few immunizations during pregnancy.
However, few randomized and large number of nonrandomized trials in which paternal lymphocyte were used for immunotherapy revealed significant obstetric outcomes in women with RSA as compared to control group where no immunotherapy was given or other sources of lymphocytes were used [1, 2, 4, 7, 8, 11, 13, 15, 16, 25, 26, 32, 33, 34, 39, 46, 48, 52, 54, 57, 66, 68, 70, 72, 73, 74, 75, 76, 77, 81, 82, 86, 90, 93, 95, 96, 100, 101, 104, 109, 110, 111, 112, 113, 115]. When we analyzed the randomized trial alone we found that very few women with RSA had complete details available as to whether they seroconverted to any one of humoral antibodies (APCA, Ab2, anti-autologous TCR antibodies, MLR-Bf, PIBF). We did meta analysis of various randomized and nonrandomized clinical trials using paternal lymphocytes for immunotherapy in women with RSA as a study group where autologous lymphocytes, third party lymphocytes, normal saline was received and also the women who did not received any kind of treatment. When we compared the success rate in randomized trials [14, 16, 26, 28, 34, 46, 47, 51, 63, 75, 77, 82, 95] we found 68% success rate in the study group as compared to a 54% success rate in women with RSA of control group, which was statistically significant (p<0.02). When the results of the randomized and nonrandomized studies were pooled (Table 1), the benefit was further seen in paternal lymphocyte immunized women with RSA of study group. However, on comparing the success rate between the pooled data of study and control group of randomized and nonrandomized trials we found 67% success rate in paternal lymphocytes immunized women with RSA under study group as compared to 36% success rate in women with RSA who received autologous lymphocytes or third party lymphocytes or normal saline or who did not received any kind of treatment under control group. This difference was reached at a level of significance (p<0.05), which supports and favors the success and efficacy of paternal lymphocyte immunotherapy as a therapeutic approach for the treatment of women with RSA. However, the drawback of these studies is the small sample size and few of these trials have no control groups for comparisons. Women randomized to immunotherapy tended to be older and reported for more spontaneous abortions than those randomized to other treatment (autologous cells, third party cells, saline) or no treatment. Although these differences were not significant, we cannot exclude a potentially worse prognosis in women allocated to immunotherapy since there were older women in this group compared with the control group. It was evident from various randomized and nonrandomized trials that there are three kinds of immunotherapy with the paternal lymphocytes for the treatment of women with RSA:
-
1.
Immunization performed before pregnancy
-
2.
Immunization performed during pregnancy
-
3.
Immunization performed before and during pregnancy
In theory immunotherapy performed before pregnancy may be beneficial for preventing extremely early abortions, because the mother was already immunized when she conceives. Cowchock and Smith [24] reported a better result of immunization early in pregnancy. However, on considering the limited duration of immunotherapy efficacy, the RSA patients have to receive immunotherapy repeatedly until they become pregnant. On the other hand, immunotherapy, if performed after pregnancy is established [4] and may be efficacious in maintaining pregnancy there after but not in preventing extremely early abortions. However Maejima et al. [66] reported that immunotherapy with the paternal lymphocytes performed before and during pregnancy produces a better outcome as compared when it is performed only before pregnancy. While Kilpatrick and Liston [54] reported that 28 women of recurrent miscarriage who had live births after paternal lymphocyte immunization were followed while 16 had subsequent pregnancies without further treatment. One pregnancy was terminated and 5 others were spontaneously aborted. Success rate of immunotherapy was 67%. Additional immunotherapy is not necessary for patients who have obtained successful results after the initial immunotherapy and are positive for MLR-Bf antibodies after their first delivery [111]. Carp et al. [13] reported that the RSA women most likely to benefit from immunotherapy are the primary or secondary aborters in whom the immune parameters may change after immunization. They, however, stressed that booster immunization may be necessary to maintain seroconversion. Tamura et al. [112] reported that titer of MLR-Bf increases with progression of pregnancy. Once these blocking antibodies developed, it is helpful in the subsequent pregnancies. Where as Reznikoff et al. [96] found a positive association between seroconversion for cytotoxic antibodies and gestational success in paternal lymphocyte treated patients. They evaluated 34 patients and found that 27 (93%) of 29 patients who were seroconverted had a live child, whereas 3 (60%) of 5 who did not had a new abortion. Similarly, Carp et al. [12] reported that 27 (72%) of 89 patients who were seroconverted had gestational success and 10 (63%) of 16 of those who were not responding had again shown failure. The result of the international collaborative study and meta analysis on allogenic lymphocyte immunization for women with RSA [26] showed that success is associated with the presence of antipaternal antibodies in both treated and control patients. But, this study does not make it clear whether these antibodies are cytotoxic antibodies or MLR-Bf. However Pena et al. [86] reported that the alloimmunization induced MLR-Bf in women with RSA was not associated with successful out come of pregnancy. They have immunized 33 RSA women with paternal lymphocytes and found that 23 (80%) of 33 women with RSA had a live child, of those women with RSA having success, only 50% produced MLR-BF. Of those patients having a new loss, 5 did and 2 did not produce MLR-Bf while 2 women with RSA who also showed pregnancy failure did not produce MLR-Bf (p<0.05). Regarding the 17 patients tested for cytotoxic antibodies 4 of the 5 patients who tested positive had a new abortion, whereas 1 of 12 whose tests remained negative did not show any gestational success.
Our work in this direction to understand the maternal reactivity in RSA was based on a series of studies on 105 women with a history of at least three consecutive unexplained abortions and 60 women 15 each from all the trimesters and 15 from post partum period with a history of at least three successful pregnancies. We have demonstrated that APCA, Ab2 and MLR-Bf developed during pregnancy and after immunotherapy in women with RSA [1, 2, 3, 80]. These antibodies are specific to paternal lymphocytes [2, 3]. We have also characterized MLR-Bf from the sera of normal pregnant and post immunized women with RSA and demonstrated that it was immunoglobulin-3 (IgG 3) in nature [83]. We immunized 73 women with RSA under nonrandomized trial and 32 women with RSA under a double blind randomized trial. A closer examination of the immunotherapy revealed that the success rate of pregnancy in the open trial was 86% and 85% in the double blind randomized trial [82]. We also evaluated the efficacy of lymphocyte immunotherapy in two conditions, (once before and once both before and during pregnancy of women with RSA) and found that it was more effective when given twice once before and once during pregnancy [81].
Komlos et al. [57] reported that 7 women with RSA who were vaccinated with the paternal lymphocytes produced significant strong blocking effect of maternal serum on mixed maternal paternal lymphocyte cultures after second vaccination. The extent of the blocking effect in maternal serum and the stimulation in control serum was much higher after immunotherapy in two cases of abortions as compared to cases with normal pregnancy outcome. In addition we have reported the effect of immunomodulation on humoral immune response in women with RSA by comparing the levels of MLR-Bf before and after paternal lymphocyte immunotherapy. It is interesting to note that all 28 women with RSA registered for immunotherapy had an activity index (AI) >1.4 before immunotherapy. Following immunotherapy 23 out of 28 women with RSA developed MLR-Bf and suppressive activity consequently increased bringing down the AI to <0.5 in all 23 women with RSA. This difference in the pre-immunotherapy and post immunotherapy groups was found to be statistically significant (p<0.05). In this study, all 28 women who were registered for immunotherapy conceived within 1 to 6 months after immunotherapy. Twenty-three women (82.15%) continued the pregnancy and have delivered normal infants the remaining 5 women with RSA (17.85%) aborted again in the first trimester [2]. Immunotherapy might react to the decidual T cell recognition of trophoblast and induce a Th2 shift leading to maintenance of pregnancy. Two independent analyses of 15 clinical centers of world wide data of nine randomized trials on allogenic lymphocyte immunization for treatment of women with RSA suggested that alloimmunization may be an effective treatment [94]. However, the data from controlled trials have produced conflicting results regarding treatment effectiveness. Daya and Gunby [26] performed a subgroup analysis of data from a world wide collaborative meta-analysis using the raw data for women with primary RSA entered into randomized controlled trials of immunotherapy which suggests that allogenic immunization is an effective treatment for unexplained primary RSA when pretreatment APCA are absent. Gafter et al. [32] reported that 7 of 9 alloimmunized women with RSA became pregnant and all of them gave birth to live newborns. They reported a decrease in the secretion of the Th-1 cytokine IL-2 and IFN-γ by patient mononuclear cells while NK and LAK cells were markedly decreased. Monocyte function of IL-1, TNF-α, IL-6 and cytotoxic activity decreased concurrently with elevation in IL-10 and TGF-β2 secretion but the production of IL-12 decreased. Recently Hayakawa et al. [39] performed paternal lymphocyte immunotherapy in 12 women with RSA where he demonstrated that this therapeutic approach plays significant role in the maintenance of pregnancy by the shift from Th1 dominant to Th2 dominant status. However, Kwak et al. [61] demonstrated that immunization with paternal lymphocytes suppresses the human NK cell cytotoxicity and CD56+NK cells levels and increases the peripheral blood CD3+ T cell population in women with RSA. These results suggest that improved pregnancy success rates following immunizations may be partly related to the suppression of cell-mediated immunity, monocyte activity and NK cell activity. These data also highlight the possibility that immunotherapy with paternal lymphocytes may give better results when performed before and during pregnancy. Some investigators reported that the success rate of immunotherapy is decreased when autoimmune antibodies (antinuclear antibody, antiphospholipid antibody) and alloimmune antibodies (APCA, Ab2, MLR-Bf) are found to be already present in preimmunized women with RSA [17, 93].
Statistical analysis
In the present study, all outcomes other than live births (continuing infertility, ectopic pregnancy, therapeutic abortion, etc.) were considered to be pregnancy failures. Comparisons of groups were made with analysis of variance and two tailed paired t-test. Effectiveness was evaluated in the pooled data by means of the Mantel-Haenzel test. P value was considered to be statistically significant if p<0.02 or highly significant if p<0.05.
Immunization dose used in various clinical trials
We compared different doses and different routes of immunization in various clinical trial of paternal lymphocyte immunotherapy for women with RSA (Table 1).We found that the protection effect of immunization is dose dependent, requiring for a single immunization at least 100×106 or >100×106 cells for optimal effects. Further, there are some suggestions that the dose administered through intradermal (i.d.) and intravenous (i.v.) routes are most effective. However, lower levels of protection being produced by the subcutaneous (s.c.), intracutaneous (i.c.) and intra muscular (i.m.) routes. Illeni et al. [47] administered 200×106 cells and only one-third of the dose was given i.v. studies reporting a favorable effect of immunotherapy used high doses but a lower dose was also used in the studies showing favorable results with immunotherapy [16]. The number of lymphocytes used in immunotherapy might be critical. Less than 60–150×106 total lymphocytes may result in a suboptimal effect, where as excessive lymphocytes (>500×106) may not be effective [16, 101]. Matsubayashi et al. [72] used 245×106 and 186×106 lymphocytes irrespectively in miscarriage and the successful group. Recent negative results of Ober et al. [77], 200×106 lymphocytes were injected at one time and patients not pregnant were immunized at 6 months interval. In a recent positive report of Orgad et al. [79] have reported that 80X106 lymphocytes were injected twice (160×106) these findings suggested that the efficacy of immunotherapy may be dependent on how many times performed and how many lymphocytes injected into the women with RSA.
Risks of immunization
The risk of immunization includes transmission of infectious organisms such as the cytomegalovirus, hepatitis B and C viruses and the human immunodeficiency viruses. However, these can also be spread by blood transfusion or use of blood products. The lymphocyte cell suspension should be adequately screened for these infections. It is important to explain the risks involved to the patient while obtaining consent for the procedure, however, adverse side effects caused by lymphocyte immunization, which occur in approximately 1 in 50 treated women [94], are concerned as some are potentially life threatening to the mother and her baby, e.g., hypotension, headache and nausea, etc.
Perlman et al. [85] reported a case of neonatal alloimmune thrombocytopenia after maternal immunization with paternal mononuclear cells. The recurrent miscarriage immunotherapy trialists group reported two cases of neonatal thrombocytopenia out of 1,149 infants whose mothers were treated by allogenic lymphocytes immunization for their unexplained recurrent abortion. However, a recent study present a very rare case of transient neonatal thrombocytopenia found in infant who was delivered by an aborter immunized with paternal lymphocytes once before pregnancy and twice at the 5th and 6th week of gestation for her successful pregnancy. Serological examination revealed that the thrombocytopenia was caused by maternal anti HLA antibodies (anti HLA IgG), which are easily absorbed by various fetal tissues, and its production was induced or enhanced by the paternal lymphocytes immunization [114].
The white cell suspension used for immunization often contains erythrocytes, which may immunize women against paternal blood groups. However, this may happen during normal pregnancy even without immunization. In early pregnancy approximately 50% of women experience vaginal bleeding despite the presence of a fetal heart beat and immunized women have a 7% incidence of intra uterine growth retardation (IUGR) compared to 14% in control patients. There is only 10% incidence of IUGR upon immunizations compared to 30% in control subjects [31]. Therefore, there is evidence that immunization may prevent rather than cause growth retardation. Preterm labor may occur in immunized women.
Conclusion
Alloimmune reproductive failures in women with RSA are psychologically and economically stressful to the childbearing population. The etiology of alloimmune causes of RSA in the vast majority of the cases is not known. However, many recent attempts have been made to understand the role and mechanism of paternal lymphocyte immunotherapy for the treatment of women with RSA. Paternal lymphocyte immunotherapy increased the expression of blocking antibodies (APCA, anti-autologous TCR antibodies, Ab2, MLR-Bf, PIBF) and suppressed Th-1 cytokines and NK cell activity. Research efforts will focus on clarifying the role of blocking antibodies, NK cell, Th1–Th2 cytokines level and their combined effect at the feto-maternal interface.
References
Agrawal S, Kishore R, Halder A, Sharma A, Sharma RK, Das V, Shukla BR, Agarwal SS (1995) Outcome of pregnancy in women with recurrent spontaneous abortion following immunotherapy with allogeneic lymphocytes. Hum Reprod 10:2280–2284
Agrawal S, Pandey MK, Pandey A (2000) Prevalence of MLR blocking antibodies before and after immunotherapy. J Hematother Stem Cell Res 9:257–262
Agrawal S, Pandey M, Mandal SK, Mishra LC, Agarwal SS (2002) Humoral immune response to an allogenic foetus in normal fertile women and recurrent aborters. BMC Pregnancy Childbirth 2:6
Aoki K, Kajiura S, Matsumoto Y, Yagani Y (1993) Clinical Evaluation of immunotherapy in early pregnancy with X-irradiated paternal mononuclear cells for primary and recurrent aborters. Am J Obstet Gynecol 169:649–653
Arck P, Dietl J, Clark D (1999) From the decidual cell internet: trophoblast-recognizing T cells. Biol Reprod 60:227–233
Beaman K, Angkachatchai V, Gilman-Sachs A (1996) TJ6: the pregnancy-associated cytokine. Am J Reprod Immunol 35:338–342
Beer AE (1984) How did your mother not reject you? Ann Immunol (Paris) 135D:315–318
Beer AE (1986) New horizons in the diagnosis, evaluation and therapy of recurrent spontaneous abortion. Clin Obstet Gynaecol 1:115–124
Beer AE, Semprini AE, Zhu XY, Quebbeman JF (1985) Pregnancy outcome in human couples with recurrent spontaneous abortions: HLA antigen profiles: HLA antigen sharing; female serum MLR blocking factors; and paternal leukocyte immunization. Exp Clin Immunogenet 2:137–153
Behar E, Carp H, Livneh A, Gazit E (1993) Differential suppression activity induced by paternal leukocyte immunization in habitual abortion. Gynecol Obstet Invest 36:202–207
Carp HJ, Toder V, Gazit E, Orgad S, Mashiach S, Nebel L, Serr DM (1990) Immunization by paternal leukocytes for prevention of primary habitual abortion: results of a matched controlled trial. Gynecol Obstet Invest 29:16–21
Carp HJ, Toder V, Mashiach S (1992) Immunotherapy of habitual abortion. Am J Reprod Immunol 28:281–284
Carp HJ, Toder V, Torchinsky A, Portuguese S, Lipitz S, Gazit E, Mashiach S (1997) Allogenic leukocyte immunization after five or more miscarriages. Recurrent Miscarriage Immunotherapy Trialists Group. Hum Reprod 12:250–255
Cauchi MN, Lim D, Young DE, Kloss M, Pepperell RJ (1991) Treatment of recurrent aborters by immunization with paternal cells—controlled trial. Am J Reprod Immunol 25:16–17
Check JH, Arwitz M, Gross J, Peymer M, Szekeres-Bartho J (1997) Lymphocyte immunotherapy (LI) increases serum levels of progesterone induced blocking factor (PIBF). Am J Reprod Immunol 37:17–20
Clark DA, Daya S (1991) Trials and tribulation in the treatment of recurrent spontaneous abortion. Am J Reprod Immunol 25:18–24
Clark DA, Arck PC, Jalali R, Merali FS, Manuel J, Chaouat G, Underwood JL, Mowbray JF (1996) Psycho-neuro-cytokine/endocrine pathways in immunoregulation during pregnancy. Am J Reprod Immunol 35:330–337
Clark DA, Arck PC, Chaouat G (1999) Why did your mother reject you? Immunogenetic determinants of the response to environmental selective pressure expressed at the uterine level. Am J Reprod Immunol 41:45–62
Clifford K, Rai R, Watson H, Regan L (1994) An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod 9:1328–1332
Coffman RL, Romagnani S (1999) Redirection of Th1 and Th2 responses. Springer, Berlin Heidelberg New York
Coulam CB (1993) Report from the Ethics Committee for Immunotherapy. Am J Reprod Immunol 30:45–47
Coulam CB, Goodman C, Roussev RG, Thomanson EJ, Beaman KD (1995) Systemic CD 56+ cells can predict pregnancy outcome. Am J Reprod Immunol 33:40–46
Coulam CB, Stephenson M, Stern JJ, Clark DA (1996) Immunotherapy for recurrent pregnancy loss: analysis of results from clinical trials. Am J Reprod Immunol 35:352–359
Cowchock FS, Smith JB (1995) Fertility among women with recurrent spontaneous abortions—the effect of paternal cell immunization treatment. Am J Reprod Immunol 33:176–181
Cowchock FS, Smith JB, David S, Scher J, Batzer F, Corson S (1990) Paternal mononuclear cell immunization therapy for repeated miscarriage: predictive variables for pregnancy success. Am J Reprod Immunol 22:12–17
Daya S, Gunby J (1994) The effectiveness of allogeneic leukocyte immunization in unexplained primary recurrent spontaneous abortion. Recurrent Miscarriage Immunotherapy Trialists Group. Am J Reprod Immunol 32:294–302
Dorling A, Monk N, Lechler R (2000) HLA-G inhibits the transendothelial cell migration of human NK cells: a strategy for inhibiting xenograft rejection. Transplant Proc 2:938
Dupont E, Moriaux M, Lambermont M, Englert Y (1998) Reevaluation of immunomodulator treatment for recurrent abortion. Rev Med Brux 19:69–72
Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ (1982) Early embryonic mortality in women. Fertil Steril 38:447–453
Ellis SA, Palmer MS, McMichael AJ (1990) Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol 44:731–735
Erard F, Wild MT, Garcia-Sanz JA, Le Gros G (1993) Switch of CD8 T cells to noncytolytic CD8-CD4-cells that make TH2 cytokines and help B cells. Science 260:1802–1805
Gafter U, Sredni B, Segal J, Kalechman Y (1997) Suppressed cell-mediated immunity and monocyte and natural killer cell activity following allogeneic immunization of women with spontaneous recurrent abortion. J Clin Immunol 17:408–419
Gatenby PA, Moore H, Cameron K, Doran TJ, Adelstein S (1989) Treatment of recurrent spontaneous abortion by immunization with paternal lymphocytes: correlates with outcome. Am J Reprod Immunol 19:21–27
Gatenby PA, Cameron K, Simes RJ, Adelstein S, Bennett MJ, Jansen RP, Shearman RP, Stewart GJ, Whittle M, Doran TJ (1993) Treatment of recurrent spontaneous abortion by immunization with paternal lymphocytes: results of a controlled trial. Am J Reprod Immunol 29:88–94
Haddad EK, Duclos AJ, Lapp WS, Baines MG (1997) Early embryo loss is associated with the prior expression of macrophage activation markers in the decidua. J Immunol 158:4886–4892
Hanna N, Hanna I, Hleb M, Wanger E, Dougherty Balkundi D, Padbury J, Sharma S (2000) Gestational age-dependent expression of IL 10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol 164:5721–5728
Harlap S, Shiono PH (1980) Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet 2:173–176
Hayakawa S, Nagai N, Kanaeda T, Karasaki-Suzuki M, Ishii M, Chishima F, Satoh K (1999) Interleukin-12 augments cytolytic activity of peripheral and decidual lymphocytes against choriocarcinoma cell lines and primary culture human placental trophoblasts. Am J Reprod Immunol 41:320–329
Hayakawa S, Karasaki-Suzuki M, Itoh T, Ishii M, Kanaeda T, Nagai N, Takahashi-Yamamoto N, Tochigi M, Chishima F, Fujii TK, Oyama J, Kitanaka S, Satoh K (2000) Effects of paternal lymphocyte immunization on peripheral Th1/Th2 balance and TCR V beta and V gamma repertoire usage of patients with recurrent spontaneous abortions. Am J Reprod Immunol 43:107–115
Haynes MK, Smith JB (1997) Can Th1-like immune responses explain the immunopathology of recurrent spontaneous miscarriage? J Reprod Immunol 35:65–71
Higuchi K, Aoki K, Kimbara T, Hosoi N, Yamamoto T, Okada H (1995) Suppression of natural killer cell activity by monocytes following immunotherapy for recurrent spontaneous aborters. Am J Reprod Immunol 33:221–227
Hill JA, Hsia S, Doran DM, Bryans CI (1986) Natural killer cell activity and antibody dependent cell-mediated cytotoxicity in preeclampsia. J Reprod Immunol 9:205–212
Hill JA, Polgar K, Harlow BL, Anderson DJ (1992) Evidence of embryo- and trophoblast-toxic cellular immune responses in women with recurrent spontaneous abortion. Am J Obstet Gynecol 166:1044–1052
Hill JA, Polgar K, Anderson DJ (1995) T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA 273:1933–1936
Hill JA III, Choi BC (2000) Immunodystrophism evidence for a novel alloimmune hypothesis for recurrent pregnancy loss involving Th1-type immunity to trophoblast. Semin Reprod Med 18:401–405
Ho HN, Gill TJ III, Hsieh HJ, Jiang JJ, Lee TY, Hsieh CY (1991) Immunotherapy for recurrent spontaneous abortions in a Chinese population. Am J Reprod Immunol 25:10–15
Illeni MT, Marelli G, Parazzini F, Acaia B, Bocciolone L, Bontempelli M, Faden D, Fedele L, Maffeis A, Radici E (1994) Immunotherapy and recurrent abortion: a randomized clinical trial. Hum Reprod 9:1247–1249
Ito K, Tanaka T, Tsutsumi N, Obata F, Kashiwagi N (1999) Possible mechanisms of immunotherapy for maintaining pregnancy in recurrent spontaneous aborters: analysis of anti-idiotypic antibodies directed against autologous T-cell receptors. Hum Reprod 14:650–655
Jaffe R, Jauniaux E, Hustin J (1997) Maternal circulation in the first-trimester human placenta—myth or reality? Am J Obstet Gynecol 176:695–705
Jalali GR, Arck P, Surridge S, Markert U, Chaouat G, Clark DA, Underwood JL, Mowbray JF (1996) Immunosuppressive properties of monoclonal antibodies and human polyclonal alloantibodies to the R80 K protein of trophoblast. Am J Reprod Immunol 36:129–134
Katano K, Ogasawara M, Aoyama J, Ozaki Y, Kajikura S, Aooki K (1997) Clinical trial of immuno-stimulation with a biological response modifier in unexplained recurrent spontaneous abortion patients. J Clin Immunol 17:427–437
Katano K, Aoki K, Ogasawara MS, Suzumori K (2000) Adverse influence of numbers of previous miscarriages on results of paternal lymphocyte immunization in patients with recurrent spontaneous abortions. Am J Reprod Immunol 44:289–292
Kilpatrick DC (1992) Soluble interleukin-2 receptors in recurrent miscarriage and the effect of leukocyte immunotherapy. Immunol Lett 34:201–206
Kilpatrick DC, Liston WA (1994) Remote pregnancy outcome after leucocyte. Immunother Fertil Steril 62:409–411
King A, Hiby SE, Verma S, Burrows T, Gardner L, Loke YW (1997) Uterine NK cells and trophoblast HLA class I molecules. Am J Reprod Immunol 37:459–462
Kishore R, Agrawal S, Halder A, Das V, Shukla BR (1996) HLA sharing, anti-paternal cytotoxic antibodies and MLR blocking factors in women with recurrent spontaneous abortion. J Obstet Gynaecol Res 22:177–183
Komlos L, Vardimon D, Notmann J, Ben-Rafael Z, Hart J, Klein T, Levinsky H, Halbrecht I (1996) Mixed maternal-paternal lymphocyte cultures before and after immunotherapy for recurrent spontaneous abortions. Am J Reprod Immunol 35:30–33
Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R (1990) A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220–223
Kruse N, Greif M, Moriabadi NF, Marx L, Toyaka KV, Rieckmann (2000) Variations in cytokine mRNA expression during normal pregnancy. Clin Exp Immunol 119:317–322
Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE (1995) Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol 34:93–99
Kwak JY, Gilman-Sachs A, Moretti M, Beaman KD, Beer AE (1998) Natural killer cell cytotoxicity and paternal lymphocyte immunization in women with recurrent spontaneous abortions. Am J Reprod Immunol 40:352–358
Lea RG, al-Sharekh N, Tulppala M, Critchley HO (1997) The immunolocalization of bcl-2 at the maternal-fetal interface in healthy and failing pregnancies. Hum Reprod 12:153–158
Li D, Li C, Zhu Y (1998) [Comparative study of the third party and paternal leukocyte immunization in recurrent spontaneous abortion of lowered maternal-fetal immuno-recognition]. Zhonghua Fu Chan Ke Za Zhi 33:597–600
Lim KJ, Odukoya OA, Ajjan RA, Li TC, Weetman AP, Cooke ID (2000) The role of T-helper cytokines in human reproduction. Fertil Steril 73:136–142
Lubinski J, Vrdoljak VJ, Beaman KD, Kwak JY, Beer AE, Gilman-Sachs A (1993) Characterization of antibodies induced by paternal lymphocyte immunization in couples with recurrent spontaneous abortion. J Reprod Immunol 24:81–96
Maejima M, Fujii T, Yamashita T, Hara N, Hamai Y, Miki A, Kozuma S, Okai T, Shibata Y, Taketani Y (1998) Immunotherapy before and during pregnancy improves pregnancy outcome in women who suffer from recurrent abortion and did not benefit from immunotherapy before pregnancy. Am J Reprod Immunol 39:12–15
Malinowski A (2001) Recurrent spontaneous abortion of alloimmunologic etiology diagnosis and immunotherapy. Ginekol Pol 72:885–898
Malinowsky D, Alheim K, Chai Z, Fantuzzi G, Hasanvan H, Di Santo E, Ghezzi P, Dinarello CA, Bartfai T (1997) Hyperresponsive febrile reactions to interleukin (IL) 1alpha and IL-1beta, and altered brain cytokine mRNA and serum cytokine levels, in IL-1beta-deficient mice. Proc Natl Acad Sci USA 94:2681–2686
Mandelboim O, Pazmany L, Davis DM, Vales-Gomez M, Reyburn HT, Rybalov B, Strominger JL (1997) Multiple receptors for HLA-G on human natural killer cells. Proc Natl Acad Sci USA 94:14666–14670
Maruyama T, Makino T, Sugi T, Iwasaki K, Ozawa N, Matsubayashi H, Nozawa S (1993) Flow cytometric crossmatch and early pregnancy loss in women with a history of recurrent spontaneous abortions who underwent paternal leukocyte immunotherapy. Am J Obstet Gynecol 68:1528–1536
Masuko-Hongo K, Hayashi K, Yonamine K, Tokuyama M, Nishioka K, Kato T (2001) Disappearance of clonally expanded T cells after allogeneic leukocyte immunotherapy in peripheral blood of patients with habitual abortion. Hum Immunol 62:1111–1121
Matsubayashi H, Maruyama T, Ozawa N, Izumi SI, Sugi T, Yoshikata K, Yoshimura Y, Makino T (2000) Anti-paternal antibodies by flow cytometry in the management of alloimmunization on recurrent miscarriages. Am J Reprod Immunol 44:284–288
Miki A, Fujii T, Ishikawa Y, Hamai Y, Yamashita T, Tadokoro K, Kozuma S, Juji T, Taketani Y (2000) Immunotherapy prevents recurrent abortion without influencing natural killer receptor status. Am J Reprod Immunol 43:98–106
Mowbray JF (1988) Immunology of early pregnancy. Hum Reprod 3:79–82
Mowbray JF, Gibbings C, Liddell H, Reginald PW, Underwood JL, Beard RW (1985) Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet 1:941–943
Mowbray JF, Underwood JL, Michel M, Forbes PB, Beard RW (1987) Immunisation with paternal lymphocytes in women with recurrent miscarriage. Lancet 2:679–680
Ober C, Karrison T, Odem RR, Barnes RB, Branch DW, Stephenson MD, Baron B, Walker MA, Scott JR, Schreiber JR (1999) Mononuclear-cell immunisation in prevention of recurrent miscarriages: a randomised trial. Lancet 354:365–369
Ohno M, Maeda T, Matsunobu A (1999) A cytogenetic study of spontaneous abortions with direct analysis of chorionic villi [review]. Obstet Gynecol 177:394–398
Orgad S, Loewenthal R, Gazit E, Sadetzki S, Novikov I, Carp H (1999) The prognostic value of anti-paternal antibodies and leukocyte immunizations on the proportion of live births in couples with consecutive recurrent miscarriages. Hum Reprod 14:2974–2979
Pandey MK, Agrawal S (2002) Prevalence of anti-idiotypic antibodies in pregnancy v/s recurrent spontaneous abortion (RSA) women. Obstet Gynaecol Today 10:574–578
Pandey MK, Agrawal S (2003) Is allogenic immunization before and during pregnancy more effective? Internet J Gynecol Obstet 2 (1)
Pandey MK, Halder A, Agarwal S, Srivastava M, Agarwal SS, Agrawal S (2003) Immunotherapy in recurrent spontaneous abortion: randomized and non-randomized trials. Internet J Gynecol Obstet 2 (1)
Pandey MK, Saxena V, Agrawal S (2003) Characterization of mixed lymphocyte reaction blocking antibodies (MLR-Bf) in human pregnancy. BMC Pregnancy Childbirth 3:2
Pazmany L, Mandelboim O, Vales-Gomez M, Davis DM, Becker TC, Reyburn HT, Seebach JD, Hill JA, Strominger JL (1999) Human leucocyte antigen-G and its recognition by natural killer cells. J Reprod Immunol 43:127–137
Pearlman SA, Meek RS, Cowchock FS, Smith JB, McFarland J, Aster RH (1992) Neonatal alloimmune thrombocytopenia after maternal immunization with paternal mononuclear cells: successful treatment with intravenous gamma globulin. Am J Perinatal 9:448–451
Pena RB, Cadavid AP, Botero JH, Garcia GP, Gallego MI, Ossa JE (1998) The production of MLR-blocking factors after lymphocyte immunotherapy for RSA does not predict the outcome of pregnancy. Am J Reprod Immunol 39:120–124
Petri M (1997) Pathogenesis and treatment of the antiphospholipid antibody syndrome. Med Clin North Am 81:151–177
Polgar K, Yacono PW, Golan DE, Hill JA (1996) Immune interferon gamma inhibits translational mobility of a plasma membrane protein in preimplantation stage mouse embryos: a T-helper 1 mechanism for immunologic reproductive failure. Am J Obstet Gynecol 174:282–287
Polifka JE, Friedman JM (1991) Environmental toxins and recurrent pregnancy loss. Infertil Reprod Med Clin North Am 2:175–213
Prigoshin N, Tambutti ML, Redal MA, Gorgorza S, Lancuba SM, Nicholson R, Testa R (1999) Microchimerism and blocking activity in women with recurrent spontaneous abortion (RSA) after alloimmunization with the partner’s lymphocytes. J Reprod Immunol 44:41–54
Raghupathy R (1997) Maternal anti-placental cell-mediated reactivity and spontaneous abortions. Am J Reprod Immunol 37:478–484
Raghupathy R, Makhseed M, Azizieh F, Omu A, Gupta M, Farhat R (2000) Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod 15:713–718
Ramhorst R, Agriello E, Zittermann S, Pando M, Larriba J, Irigoyen M, Cortelezzi M, Auge L, Lombardi E, Etchepareborda JJ, Contreras Ortiz C, Fainboim L (2000) Is the paternal mononuclear cells’ immunization a successful treatment for recurrent spontaneous abortion? Am J Reprod Immunol 44:129–135
Recurrent Miscarriage Immunotherapy Trialists Group (RMITG) (1994) World wide collaborative observation study and meta-analysis on allogenic leukocyte immunotherapy for recurrent spontaneous abortion. Am J Reprod Immunol 32:55–72
Regan L, Braude PR, Trembath PL (1989) Influence of past reproductive performance on risk of spontaneous abortion. BMJ 299:541–545
Reznikoff-Etievant MF, Durieux I, Huchet J, Slamon C, Neltor A (1988) Human MHC antigens and paternal leukocyte injections in recurrent spontaneous abortions. In: Bear RW, Sharp F (eds) Early pregnancy loss: mechanisms and treatment. RCOG, London, pp 375–384
Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED (1997) The alpha1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc Natl Acad Sci USA 94:5249–5254
Saito S (2000) Cytokine network at the feto-maternal interface. J Reprod Immunol 47:87–103
Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, Morikawa H (1999) Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol 41:297–306
Smith JB, Cowchock FS (1988) Immunological studies in recurrent spontaneous abortion: effects of immunization of women with paternal mononuclear cells on lymphocytotoxic and mixed lymphocyte reaction blocking antibodies and correlation with sharing of HLA and pregnancy outcome. J Reprod Immunol 14:99–113
Smith JB, Cowchock FS, Lata JA, Hankinson BT (1992) The number of cells used for immunotherapy of repeated spontaneous abortion influences pregnancy outcome. J Reprod Immunol 22:217–224
Stephensen MD (1996) Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 66:24–29
Stern JJ, Dorfmann AD, Gutierrez-Najar AJ, Cerrillo M, Coulam CB (1996) Frequency of abnormal karyotypes among abortuses from women with and without a history of recurrent spontaneous abortion. Fertil Steril 65:250–253
Sterzik K, Strehler E, De Santo M, Oblinger E, Rosenbusch B, Kreienberg R (1995) Idiopathic habitual abortion: experiences with active immunotherapy. Geburtshilfe Frauenheilkd 55:493–499
Strobino B, Warburton D (1995) Recurrent abortion: genetic and other non immune factors. In: Reed GB, Wairnex AE, Cockburn F (eds) Diseases of the foetus and newborn, pathology, imaging, genetics and management, 2nd edn. Chapman and Hall, London, pp 167
Sugi T, Makino T, Maruyama T, Nozawa S, Iizuka R (1993) Influence of immunotherapy on antisperm antibody titer in unexplained recurrent aborters. Am J Reprod Immunol 29:95–99
Szpakowski A, Malinowski A, Glowacka E, Wilczynski JR, Kolasa D, Dynski M, Tchorzewski H, Zeman K, Szpakowski M (2000) [The influence of paternal lymphocyte immunization on the balance of Th1/Th2 type reactivity in women with unexplained recurrent spontaneous abortion]. Ginekol Pol 71:586–592
Szpakowski A, Malinowski A, Zeman K, Wilczynski J, Kolasa D, Nowak M, Wladzinski J, Kaminski T, Szpakowski M (2001) [The influence of paternal lymphocytes immunization on percentage of peripheral blood CD16+/CD56+ cells in women with primary recurrent spontaneous abortion]. Ginekol Pol 72:1063–1068
Takakuwa K, Kanazawa K, Takeuchi S (1986) Production of blocking antibodies by vaccination with husband’s lymphocytes in unexplained recurrent aborters: the role in successful pregnancy. Am J Reprod Immunol Microbiol 10:1–9
Takakuwa K, Goto S, Hasegawa I, Ueda H, Kanazawa K, Takeuchi S, Tanaka K (1990) Result of immunotherapy on patients with unexplained recurrent abortion: a beneficial treatment for patients with negative blocking antibodies. Am J Reprod Immunol 23:37–41
Takakuwa K, Higashino M, Yasuda M, Ishii S, Ueda H, Asano K, Kazama Y, Tanaka K (1993) Is an additional vaccination necessary for a successful second pregnancy in unexplained recurrent aborters who were successfully immunized with their husband’s lymphocytes before the first pregnancy? Am J Reprod Immunol 29:39–44
Tamura M, Takakuwa K, Arakawa M, Yasuda M, Kazama Y, Tanaka K (1998) Relationship between MLR blocking antibodies and the outcome of the third pregnancy in patients with two consecutive spontaneous abortions. J Perinat Med 26:49–53
Tanaka T, Umesaki N, Maeda K, Miyama M, Ogita S (1997) Pregnancy and neonatal outcomes in unexplained recurrent/habitual aborters treated by allogenic leukocyte immunization. Osaka City Med J 43:81–87
Tanaka T, Umesaki N, Nishio J, Maeda K, Kawamura T, Araki N, Ogita S (2000) Neonatal thrombocytopenia induced by maternal anti-HLA antibodies: a potential side effect of allogenic leukocyte immunization for unexplained recurrent aborters. J Reprod Immunol 46:51–57
Taylor C, Faulk WP (1981) Prevention of recurrent abortion with leucocyte transfusions. Lancet 2:68–70
Torpin R (1995) Placenta circumvallata and placenta marginata. Obstet Gynecol 21:76–81
Warburton D, Fraser FC (1961) On the probability that a woman who has had a spontaneous abortion will abort in subsequent pregnancies. Br J Obstet Gynecol 69:784–787
Warburton D, Kline J, Stein Z, Hutzler M, Chin A, Hassold T (1983) Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet 41:465–483
Wegmann TG, Lin H, Guilbert L, Mosmann TR (1993) Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14:353–356
Weltz JI (1997) Low molecular weight heparins. N Engl J Med 337:688–697
Wilson R, McInees I, Leung B, McKillop JH, Walker JJ (1997) Altered interleukin 12 and nitric oxide levels in recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol 75:211–214
Yamada H, Kato EH, Kobashi G, Ebina Y, Shimada S, Morikawa M, Sakuraji N, Fugimoto S (2001) High NK cell activity in early pregnancy correlates with subsequent abortion with normal chromosomes in women with recurrent abortion. Am J Reprod Immunol 46:132–136
Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ (1994) Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta 15:819–835
Zenclussen AC, Kortebani G, Mazzolli A, Margni R, Malan Borel I (2000) Interleukin-6 and soluble interleukin-6 receptor serum levels in recurrent spontaneous abortion women immunized with paternal white cells. Am J Reprod Immunol 44:22–29
Zenclussen AC, Fest S, Busse P, Joachim R, Klapp BF, Arck PC (2002) Questioning the Th1/Th2 paradigm in reproduction: peripheral levels of IL-12 are down-regulated in miscarriage patients. Am J Reprod Immunol 48:245–251
Acknowledgements
The authors thank the Indian Council of Medical Research (ICMR) and Council of Science and Industrial Research (CSIR) India for financial support of their laboratory work. Boyd Karen for proof reading, Sanjay Kumar Johari and Mukesh Srivastava for their expert advice in computers and statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, M.K., Thakur, S. & Agrawal, S. Lymphocyte immunotherapy and its probable mechanism in the maintenance of pregnancy in women with recurrent spontaneous abortion. Arch Gynecol Obstet 269, 161–172 (2004). https://doi.org/10.1007/s00404-003-0560-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-003-0560-3