Abstract
Dermatitis is a group of highly pruritic chronic inflammatory skin diseases which represents a major public-health problem worldwide. The prevalence of dermatitis has increased in recent years affecting up to 20% of the general population. Acute skin lesions are characterized by extensive degrees of intercellular edema of the epidermis (spongiosis) and a marked perivenular inflammatory cell infiltrate in the dermis. Keratinocytes within eczematous lesions exhibit a modified expression of proinflammatory cytokines, chemokines and cell-surface molecules. The pathophysiological puzzle of dermatitis is far from being elucidated completely, but skin infiltration of activated memory/effector T cells are thought to play the pivotal role in the pathogeneses. The aim of this study was the set-up of organotypic models mimicking the symptoms of eczematous dermatitis to provide a tool for therapeutic research in vitro. Therefore activated T cells (ATs) were integrated in organotypic skin and epidermis equivalents (SE, EE). These models enabled the reproduction of several clinical hallmarks of eczematous dermatitis: (1) T cells induce keratinocyte apoptosis, which leads to a reduced expression of the adhesion molecule E-cadherin (E-cad) and disruption of the epidermal barrier. (2) Expression of intercellular adhesion molecule-1 (ICAM-1) allows the attachment of leukocytes to epidermal cells. (3) Upregulation of neurotrophin-4 (NT-4) in the epidermis is thought to mediate pruritus in lesions by supporting nerve outgrowth. (4) Elevated levels of pro-inflammatory cytokines (IL-1α and IL-6) and chemokines (IL-8, IP-10, TARC, MCP-1, RANTES and eotaxin) amplify the inflammatory response and lead to an influx of secondary immunocells into the skin. The therapeutics dexamethasone and FK506 markedly reduce cytokines/chemokines production and epidermal damaging in these models. These data underline that activated memory/effector T cells induce eczematous changes in this HaCaT cell based organotypic skin equivalent. Furthermore it can be concluded that these models make it possible to investigate targets of therapeutics in skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eczematous dermatitis, such as atopic dermatitis and allergic contact dermatitis, comprises a heterogeneous group of skin diseases characterized by typical clinical features include itching, redness, papules and scaling. Atopic dermatitis, which affects up to 20% of the general population [23], usually develops in early childhood and is frequently seen in children with a personal history of respiratory allergy and/or a family history of atopic disease [24]. However, acute skin lesions are characterized by extensive degrees of intercellular edema of the epidermis (spongiosis) and a marked perivenular inflammatory cell infiltrate in the dermis. Keratinocytes within eczematous lesions exhibit a modified expression of proinflammatory cytokines, chemokines, and cell-surface molecules.
The eczema formation is thought to be mainly related to the accumulation of activated memory/effector (CD45RO+) T cells, bearing the cutaneous lymphocyte-associated antigen (CLA) [9, 37]. In addition, T cells mediate epidermal barrier disruption [42] and are known to enhance IgE production and to induce eosinophilia. A number of pathogenetic mechanisms lead to T cell activation including aeroallergens, food allergens and superantigens followed by skin-selective homing [6, 25, 38]. Given the complexity of the inflammatory cascades that lead to eczema, a multipronged approach is required for successful treatment. Besides skin hydration and elimination of exacerbating factors like irritants, allergens, emotional stress and infectious agents [14] topical glucocorticosteroid (e.g. dexamethasone) application is still the reference standard of anti-inflammatory treatment of eczematous dermatitis [26]. Tacrolimus is an effective topical alternative to glucocorticosteroids, to reduce clinical symptoms [4].
Several skin equivalents have been developed to provide useful models for investigations of skin physiology, used for toxicological and pharmacological investigations. In this study activated CD45RO+ T cells were integrated into the three-dimensional structure of skin equivalents according to atopic eczematous skin lesions. In a second in vitro model, T cells were cocultured with epidermis equivalents. The use of the spontaneously immortalized keratinocyte cell line HaCaT [5] in these equivalents instead of primary keratinocytes assumed a better standardization due to unlimited availability and avoidance of genetic and morphological interindividual variability. HaCaT cells have often been used in reconstructed skin equivalents, e.g., in combination with human melanocytes [41]. The aim of the present study was the reproduction of several T-cell-induced hallmarks of eczema using the two newly developed skin models. In this context the effects of different therapeutics were investigated.
Materials and methods
Reagents and antibodies
Chondroitin-6-sulphate was obtained from Fluka (Buchs, Switzerland), chondroitin-4-sulphate, cholera toxin, hydrocortisone, and dexamethasone were purchased from Sigma (Deisenhofen, Germany). Tacrolimus was obtained from Calbiochem (San Diego, USA), collagenase typ I and dispase were obtained from Invitrogen (Karlsruhe, Germany). Rat tail collagen type I was purchased from ARS-ARTHRO (Esslingen, Germany), and fibronectin was purchased from Roche Molecular Biochemicals (Mannheim, Germany). The following antibodies were used: anti-E-cad, anti-ICAM-1, anti-NT-4 (all from Santa Cruz Biotechnology, Heidelberg, Germany), biotin-conjugated anti-rabbit IgG (DakoCytomation, Hamburg, Germany), anti-CD2 (6F10.3 and 6F10.3, Immunotech, Marseille, France), anti-CD3 and anti-CD28 (both from BD Biosciences, Heidelberg, Germany).
Isolation of human fibroblasts
Human foreskin fibroblasts were obtained from ten different donors; the age ranged from 3 years to 8 years. Appropriate informed consent was obtained for all subjects, and the study was approved by the local Ethical Committee (Landesärztekammer BW, Stuttgart). The skin-biopsies were washed, cut into pieces of 10 mm2 and incubated overnight at 4°C in 20 mg/ml dispase prepared in PBS− (phosphate buffered saline). The following day, the epidermis was removed and the dermal tissue was minced and incubated for 45 min at 37°C in 0.25% collagenase prepared in PBS+ (phosphate buffered saline with calcium and magnesium). Thereafter cells were cultured in DMEM (10% FCS), pooled and stored in liquid nitrogen until use.
Isolation of CD45RO+ T cells
Mononuclear cells from human peripheral blood were obtained by Ficoll-Paque Plus (Amersham Pharmacia Biotech, Freiburg, Germany) density gradient centrifugation.
Blood samples were collected from two healthy volunteers. CD45RO+ T cells were isolated with the MACS system according to the instructions of the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany). In brief, non- and naive (CD45RA+) T cells were depleted with the Pan T Cell Isolation Kit and CD45RA Microbeads.
Activation of memory-effector (CD45RO+) T cells
Isolated CD45RO+ T cells cultured in RPMI 1640 (10% FCS, 25 mM HEPES) were activated with a combination of soluble anti-CD3 (0.2 μg/ml), anti-CD2 (1 μg/ml) and anti-CD28 (5 μg/ml) for 12 h before inserting into the equivalent. Successful activation of the T cells was monitored by cluster formation (data not shown).
Skin equivalents (SE)
Dermal equivalents were made from fibroblast cultures at passage two. When confluency was attained, fibroblasts were harvested and suspended in neutralization-solution (50 vol% 5×DMEM, 25 vol% 1 M HEPES, 25 vol% FBS, 12.5 μg/100 ml chondroitin-4-sulphate, 12.5 μg/100 ml chondroitin-6-sulphate, pH 7.8) to a final concentration of 4×105 cells/ml. A collagen solution mixture was prepared by quickly mixing two volumes of rat tail collagen type I (6 mg/ml) with one volume of the cell suspension. Subsequently 600 μl of the fibroblast–collagen-mixture were transferred into 10 mm polycarbonate cell culture inserts (8.0 μm; NUNC, Wiesbaden, Germany) in 24-well culture dishes. After collagen polymerization 35 μl fibronectin solution (75 μg/ml) was pipetted on top, and the collagen gels were cultured submerged in DMEM. After 2 days, the collagen gels were coated again with fibronectin and HaCaT cells (Prof. Dr. N.E. Fusenig, German Cancer Research Center, Heidelberg, Germany) at passage 40–46 were seeded on top (1×105 cells per insert). After an additional 2 days the skin equivalents were raised to the air–liquid interface, and the so-called air-lift medium (3:1 mixture of DMEM and Ham’s nutrient mixture F12 medium supplemented with 10% FBS, 50 μg/ml ascorbate, 0.4 μg/ml hydrocortisone and 10−10 M cholera toxin) was added to the bottom. Media changes were performed every 2 days.
For further experiments skin equivalents, grown for 12 days at the air–liquid interface, were combined with activated or non-activated CD45RO+ T cells. During this part of the experiment, hydrocortisone and cholera toxin were omitted from the culture medium. First new cell culture inserts were coated with 100 μl cell-free collagen solution mixture, next 50 μl of a mixture containing 5×105 T cells were added and allowed to become solid. Then skin equivalents were stuck on top using 50 μl cell-free collagen solution mixture as adhesive. After polymerization the equivalents were removed from the inserts and incubated for additional 3 days with RPMI (serum-free, 25 mM HEPES) in 6-well culture dishes at the air–liquid interface.
Epidermis equivalents (EE)
Fibroblasts of passage two were cultured in 12-well culture dishes in DMEM. When fibroblasts reached confluence, 12 mm polyester cell culture inserts (0.4 μm; Corning Inc., New York, USA) coated with 40 μl type I rat tail collagen (1 mg/ml) and 70 μl fibronectin (75 μg/ml), were placed into the wells. HaCaT cells at passage 40–46 were resuspended in DMEM and 2.5×105 cells in a volume of 500 μl were seeded on top. The cell culture inserts were kept submerged for 2 days; thereafter the membranes were raised to the air–liquid interface. Airlift-medium was added to the bottom and changed every day. On day 22 the membrane equivalents were removed from the fibroblast culture and cocultured with activated or non-activated CD45RO+ T cells (5×105) in 500 μl RPMI (serum-free, 25 mM HEPES) for an additional 3 days.
Treatment with the therapeutics dexamethasone and FK506
The protective effects of therapeutics on epidermal barrier damaging and cytokine production were investigated by applying them in different concentrations to EE-ATs (1 μM up to 10 μM) and SE-ATs (10 μM), respectively. Dexamethasone and FK506 were added topically as well as into the medium at the time point of co-cultivation of the different models with the activated T cells.
Trans epithelial electric resistance (TEER)
Electric resistance of membrane-skin equivalents were measured using the epithelial ohmmeter equipped with a STX3 electrode (WPI, Berlin, Germany). Two electrodes were positioned, one below the membrane in the outer well and the other above the equivalent, each submersed in PBS−. The resistance of membranes without cells was subtracted from each TEER value, and readings were expressed in ohm (Ω).
Quantification of cytokines
Cell-free supernatants were tested for cytokine content by ELISA. IL-1α, IL-6, IL-8, IP-10, MCP-1, TARC, RANTES and eotaxin concentration were quantified by Quantikine human ELISA kits (R&D Systems, Wiesbaden-Nordenstadt, Germany) according to the manufacturer’s protocol. Optical density was measured at 450 nm with Labsystems iEMS Ia Reader MF (Labsystems, Helsinki, Finland). Results are given as ng/ml ± SD.
Immunohistochemistry
Skin equivalents were frozen in liquid nitrogen, embedded in Tissue-Tek, and stored at −80°C. Sections of 10 μm were prepared with the cryotom HM560 (Microm, Walldorf, Germany) and stored at −80°C until analysis. The samples were air-dried, fixed in 4% paraformaldehyde for 20 min at room temperature. Sections were incubated with the primary antibody at 4°C overnight followed by incubation with biotin-conjugated goat anti-rabbit IgG and preformed streptABComplex alkaline phosphatase (DakoCytomation) at room temperature for 1 h. Incubation with Sigma FastRed (Sigma) was used for visualization. For control purposes, samples were stained with the second antibody only.
Detection of apoptosis
The TUNEL reaction was applied according to the instructions of the manufacturer (In Situ Cell Death Detection Kit, Roche Molecular Biochemicals) with slight modifications: the permeabilization time was reduced to 1 min, and the labeling reaction was terminated by transferring the slides to stop-buffer (300 mM sodium citrate, 30 mM NaCl) for 15 min (as originally described by Gavrieli et al. [8]).
Statistical analysis
Results are shown as means ± SD of data. Statistical analyses were performed with the Student t test.
Results
In this study two different in vitro models with clinical hallmarks of eczematous dermatitis were developed: on the one hand a skin equivalent consisting of an epidermis and a dermis part combined with activated CD45RO+ T cells; on the other hand an epidermis equivalent differing in the lack of the dermal layer. In general the following results show the comparison between equivalents with activated T cells and controls with non-activated T cells. The integration of non-activated T cells into the equivalents do not affect histological appearance or expression of tested markers compared to T cell-free equivalents (data not shown).
Activated T cells mediate epidermis injury and expression of ICAM-1 and NT-4 in skin equivalents
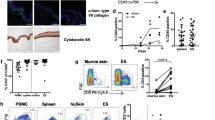
The controls show an intact, multilayered and differentiated epidermis, terminated by a horny layer. Most basal cells were cuboid in shape, as expected for basal cells in normal human skin (Fig. 1). SE with incorporated activated T cells in the dermal part (SE-AT) exhibit histological hallmarks of eczematous dermatitis. The epidermis is characterized by condensation of the HaCaT cells, widening of the intercellular space and stretching of remaining intercellular contacts, resulting in spongiosis. Furthermore an increased thickness of the epidermis can be observed (31.8 ± 7.7%). Apoptotic HaCaT cells were detectable in all epidermal layers of the SE-ATs, whereas in the controls expression of TUNEL-positive HaCaT cells was barely detectable. Furthermore in the controls staining of E-cad is clearly visible at the surface of all epidermal HaCaT cell layers and showed typical distribution. In contrast in the spongiotic epidermis of the SE-ATs E-cad staining is markedly reduced mainly in the stratum spinosum. Intercellular adhesion molecule-1 (ICAM-1) and neurotrophin-4 (NT-4) expression was found to be upregulated in the epidermis of the SE-ATs. ICAM-1 expression was localized in basal as well as in suprabasal layers, at the cell membrane with a slight cytoplasmic staining. In controls ICAM-1 expression was absent from epidermal HaCaT cells, and a weak intra- and extracellular NT-4 staining was observed. In the SE-ATs NT-4 was found to be upregulated.
Effects of activated T cells on epidermal damaging, HaCaT cell apoptosis, E-cad degradation and expression of ICAM-1 and NT-4 in skin equivalents. Frozen sections of skin equivalents containing activated T cells (SE-AT) were stained with hematoxylin/eosin (H&E), subjected to TUNEL and immunostained with antibodies against E-cad, ICAM-1 and NT-4. Skin equivalents containing non-activated T cells served as control. Scale bar: 100 μm
Effects of dexamethasone and FK506 on the integrity of the epidermal barrier
The appearance of spongiosis, which means the loss of cell–cell interactions in the epidermis, was correlated with their TEER. Thereby differences in the integrity of the epidermal barrier can be quantified leading to improved determination of effects of therapeutics. Epidermis equivalents were cocultured for 3 days with activated CD45RO+ T cells (EE-AT). We demonstrated that T-cell-induced spongiosis results in decreased electric resistance across the epidermis (Fig. 2). The protective effects of therapeutics on epidermal barrier damaging were investigated by applying them in different concentrations to EE-ATs topically and in the medium, respectively. Dexamethasone and FK506 significantly prevent the loss of cell–cell interactions in the epidermis. The effects were dose-dependent with effective concentrations in the 1–10 μM range.
Effects of dexamethasone and tacrolimus on trans epithelial electrical resistance (TEER) of epidermis equivalents. Epidermis equivalents were cocultured with activated T cells (EE-AT) and let untreated or supplemented with different concentrations of dexamethasone or tacrolimus ranging from 0.01 mmol/l to 10.0 mmol/l, respectively. TEER measurements were performed after 3 days. Results were expressed in percent related to the electric resistance of control epidermis equivalents cultured with non-activated T cells (61.3 Ω) and shown as mean ± SD (n=3). *P<0.05 compared to untreated EE-AT. **P<0.01 compared to untreated EE-AT. ***P<0.001 compared to control EE
Dexamethasone and FK506 reduce T-cell-induced cytokine production in skin equivalents
The capacity of activated T cells to induce production and release of cytokines in SEs was investigated. In order to distinguish the location of cytokine production within the skin model, fibroblasts/collagen layers containing activated T cells were used in addition to SE-AT. Because activated T cells themselves produce cytokines, the cytokine release of single collagen layers containing activated T cells was also measured. SEs with non-activated T cells served as control in order to detect basic levels of secreted cytokines. Supernatants were collected at several time points and tested for cytokine content by ELISA (IL-1α, IL-6, IL-8, IP-10, TARC, MCP-1, RANTES and eotaxin). The cytokine values at 96 h were summarized in Table 1 and Fig. 3. The controls secreted only IL-1α, IL-6, IL-8, MCP-1, TARC and eotaxin in significant levels. In SE-AT, activated T cells were able to strongly increase the release of these cytokines and induce further the secretion of IP-10, MCP-1, TARC, and RANTES in a time-dependent manner. The secretion of all cytokines increased continually until the latest time point measured (96 h). The most abundant cytokines released by SE-AT were IL-6 (999.4 ±65.8 ng/ml) and MCP-1 (1008.2 ±89.3 ng/ml). IL-8 (47.7 ±9.9 ng/ml), IP-10 (8.8 ± 0.7 ng/ml) and RANTES (49.3 ±2.0 ng/ml) were released in moderate, and IL-1α (0.23 ±0.03 ng/ml), TARC (0.10 ±0.01 ng/ml) and eotaxin (0.18 ±0.01 ng/ml) in very limited but significant amounts.
Effects of dexamethasone and FK506 on cytokine release of skin equivalents. Topic application of 10 μM dexamethasone or tacrolimus, respectively on skin equivalents containing activated cells (SE-AT). Skin equivalents containing non-activated T cells served as controls. Quantification of cytokines was performed after 96 h. Values represent the mean ng/ml ± SD (n=3). **P<0.01 compared to untreated SE-AT. ***P<0.001 compared to untreated SE-AT
The incorporation of activated T cells promoted different cytokine patterns in the epidermal compared to the dermal layer. The amounts of IP-10 and MCP-1 released in the epidermal and dermal layer were comparable. However, IL-1α and TARC were predominantly produced by HaCaT cells whereas IL-6, IL-8, RANTES, and eotaxin were mainly produced by fibroblasts. Activated T cells themselves only produced low amounts of TARC and RANTES. The effect of therapeutics on cytokine release was investigated in the following experiment by applying 10 μM dexamethasone or FK506 topically (Fig. 3). Treatment with dexamethasone caused a dramatic decrease of cytokine release. The amount of IL-1α (96% reduction at 96 h compared to elevated secretion), IP-10 (97%), MCP-1 (96%), TARC (98%) and RANTES (99%) decreased to concentrations comparable to the control, whereas IL-6 (129%), IL-8 (112%) and eotaxin (116%) decreased to even lower concentrations than the control. FK506 treatment mediates a strong decrease of MCP-1 (89%), TARC (95%) and eotaxin (102%), a moderate decrease of IL-6 (74%) and RANTES (79%) and a lower decrease of IL-1α (57%), IL-8 (56%) and IP-10 (27%).
Discussion
In this study several clinical hallmarks of eczematous dermatitis were reproduced in an organotypic skin model (SE-AT) to provide a tool for therapeutic research in vitro. Interactions between T cells and skin cells via cytokines and adhesion molecules are supposed to play a central role in the pathophysiology of atopic dermatitis and other inflammatory cutaneous disorders. In the organotypic skin model used in this study activated T cells were incorporated and allow the generation of complex cytokine cascades and networks between T cells, fibroblasts and a differentiated epidermis approximate to the in vivo situation.
In the present study we demonstrate that activated CD45RO+ T cells induce HaCaT cell apoptosis in the epidermis of our skin model. Apoptotic keratinocytes are also present in lesional skin of patients with eczematous disorders. It has been proposed by Trautmann et al. that Fas-induced keratinocyte apoptosis, caused by skin-infiltrating CD45RO+ T cells, is a major mechanism in the pathogenesis of eczematous dermatitis [42]. Our skin model exhibits spongiosis in epidermal areas of intense apoptosis. It is characterized by rounding and nucleus condensation of the HaCaT cells and widening of intercellular spaces in the epidermis. Spongiosis is the long-recognized histological hallmark of epidermis in eczema. It decreases the effectiveness of the barrier, allowing greater access of allergens and superantigens to Langerhans cells, dermal dendritic cells, and T cells, amplifying the inflammatory process. In the spongiotic epidermis of our skin model the immunohistological E-cadherin staining was markedly reduced, comparable to findings in the skin of AD patients [44]. The development of spongiosis is initiated by keratinocyte apoptosis due to cell shrinkage and cleavage of E-cadherin. In healthy skin, adherens junctions mediated by E-cadherin form strong keratinocyte–keratinocyte contacts. During the early stage of apoptosis of keratinocytes, E-cadherin is cleaved by caspases that remove the β-catenin-binding domain from its cytoplasmic tail [44].
For better quantification, the disruption of the epidermal barrier was reproduced in an epidermis model (EE-AT). In different studies it was shown that the TEER allows the characterization of intercellular contacts [28] or skin irritations [34]. We demonstrated that the degree of epidermal disruption correlates with the decline of TEER across the epidermis in this model, offering a tool to investigate protective effects of therapeutics. In this study the efficacy of the therapeutics dexamethasone and tacrolimus in inhibiting T-cell-induced damage of the epidermal barrier was investigated. The results show, that dexamethasone and tacrolimus prevent the loss of cell–cell interactions in the epidermis in a dose-dependent manner, with a higher efficiency of dexamethasone. This can be explained by the different modes of action. Dexamethasone is reported to inhibit early steps of antigen receptor signaling in activated T cells [3] and FasL expression [52]. Furthermore, it has been shown that dexamethasone protects epithelial cells from IFN-γ and anti-Fas-induced cell death. Dexamethasone inhibits caspase 3 and caspase 7 activation and therefore blocks apoptosis directly [50]. The beneficial effects of tacrolimus could be attributed mainly to the inhibition of T cell activation [17] via blocking of calcineurin activation, which is important for proper intracellular T cell signaling [32]. Tacrolimus is not able to directly inhibit keratinocyte apoptosis [43]. This may explain the greater beneficial effects of dexamethasone. Trautmann et al. postulate, that keratinocyte apoptosis induced by activated T cells is a useful parameter to evaluate the activity of eczematous dermatitis [43]. Therefore monitoring of the integrity of the epidermal barrier can indicate the severity of the lesion and the effectiveness of treatment, respectively.
In chronic inflammatory skin diseases lymphocytes infiltrate the dermis and migrate to the epidermis in an organized manner. Adhesion molecules, especially ICAM-1, are necessary for attachment to epidermal cells. It has been proposed by Nickoloff et al. that induction of ICAM-1 may be a “final common pathway” in the pathophysiology of several inflammatory skin diseases [33]. In our SE-AT model, ICAM-1 expression occurred in basal and suprabasal layers of the epidermis. This finding correlates with ICAM-1 expression in atopic dermatitis in vivo, which was observed mainly in basal cell layers adjacent to T cells infiltrating the dermis [13]. In contrast, ICAM-1 expression was absent from control skin equivalents. This finding shows that there is no alloreaction due to incorporation of T cells into the HaCaT based skin model. It is also in accordance with the observation that healthy human skin contains barely detectable ICAM-1 expressing keratinocytes [7].
A further clinical hallmark of eczematous dermatitis is the intense chronic pruritus [36]. Prurigo lesions are histologically characterized by an increased number of sensoric nerve fibers. NT-4 has been described to support nerve survival and outgrowth [16, 35] and is highly expressed in chronic inflammatory skin diseases, suggesting a pathophysiological role. In our control skin equivalents only a weak basal level of NT-4 expression in the epidermis has been observed, which is in accordance with in vivo studies demonstrating a low NT-4 expression in keratinocytes of normal human skin. In our SE-AT model we observed a strongly upregulated NT-4 expression in the epidermis. It has been reported that IFN-γ induces NT-4 expression in human keratinocytes [12]. Consequently, T cell derived IFN-γ is potentially the main inducer of NT-4 expression in our skin model. This finding underlines a close relationship between infiltrating of activated CD45RO+ T cells in eczematous dermatitis and an increased presence of nerve fibers within the skin lesions.
Activated T cells locally released lymphokines (e.g., IFN-γ, IL-2, IL-4, IL-5, IL-10 and IL-13) influence the immune functions of skin cells, including keratinocytes and fibroblasts. In the present study it was demonstrated that in the skin model HaCaT cells and fibroblasts are potent producers of the pro-inflammatory cytokines IL-1α and IL-6 and the chemokines IL-8, IP-10, TARC, MCP-1, RANTES, and eotaxin in the presence of activated T cells. The reciprocal activation of T cells and resident skin cells has a primary role in the amplification of skin inflammation during immune-mediated skin diseases. IL-1α, a cytokine produced during skin inflammation, participates in the regulation of local and systemic immunologic and inflammatory reactions [2]. IL-6 controls in synergism with IL-1 the initial step in peripheral T cell activation and proliferation [46]. IL-8 plays an important role for neutrophil, basophils and T cell recruitment in the skin [22, 51, 53]. The high levels of IL-6 and IL-8 found in our skin model combined with their growth-promoting activity on keratinocytes [20, 45] suggest that these cytokines are likely to contribute to the epidermal hyperproliferation seen in our skin model and suggest a cause for the hyperplasia in eczematous dermatitis. In accordance with the in vivo situation in normal skin conditions the secretion of IL-6 and IL-8 in control skin equivalents appears weak to moderate [1]. The elevated expression of TARC, IP-10, MCP-1, eotaxin, and RANTES in our SE-AT model is also in accordance with in vivo findings in plasma and skin of AD patients [11, 30, 39, 47]. Furthermore, Klunker et al. describe an increased IP-10-expression in primary keratinocytes in vitro after exposure to IFN-γ [19]. These findings are in accordance to Villagomez et al., who investigated whether skin fibroblasts, besides keratinocytes, are able to produce IP-10 [48]. Villagomez concluded that the main inducer of IP-10 in keratinocytes was IFN-γ, whereas TNF-α was the main inducer of IP-10 in fibroblasts [48]. These data provide a potent indication of the successful activation of the T cells in the present study and confirm our findings concerning the IP-10 expression.
Since production of cytokines in the course of eczematous dermatitis is thought to be a sequential chain of events [10, 15], this indicates that chemokines are useful immunologic indicators for monitoring disease activity in eczematous dermatitis.
Dexamethasone and tacrolimus were also used in the SE-AT model to proof the reaction of the model to known therapeutics for eczematous dermatitis on cytokine production. In the SE-AT model topic application of dexamethasone completely inhibited T-cell-induced production of all tested cytokines. In addition dexamethasone reduced the IL-6, IL-8 and eotaxin baseline levels. Treatment with tacrolimus showed a strong inhibition of MCP-1, TARC and eotaxin but only a partial inhibition of IL-1α, IL-6, IL-8, IP-10 and RANTES production. This finding suggests that dexamethasone and, in some case to a lesser extent, tacrolimus are able to suppress inflammatory cell infiltrates by inhibiting chemokine production by keratinocytes and fibroblasts. Several studies reported, that dexamethasone directly inhibits the production of different cytokines in several cell types including fibroblasts and keratinocytes [18, 21, 27, 31, 40, 49]. There are only few studies on the effects of tacrolimus on cytokine production in skin. It is known that tacrolimus inhibits production of IL-8 [29] and RANTES in keratinocytes [38]. It was also reported by Wakugawa et al. that tacrolimus inhibits cytokine-induced RANTES production by keratinocytes to a lesser extent than dexamethasone [49]. This finding is consistent with the data of the present study. Taken together it is evident, that topical dexamethasone and tacrolimus directly inhibit T-cell-induced cytokine expression in our skin model.
It can be concluded, that this skin model offers the possibility to investigate targets of various therapeutics in skin: integrity of the epidermal barrier and immune functions of skin cells. The model presented in this study can provide an opportunity for testing candidate drugs, but data obtained using immortalized cells should be interpreted with caution in terms of the effective concentration.
References
Anttila HS, Reitamo S, Erkko P, Ceska M, Moser B, Baggiolini M (1992) Interleukin-8 immunoreactivity in the skin of healthy subjects and patients with palmoplantar pustulosis and psoriasis. J Invest Dermatol 98:96–101
Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ (1991) Keratinocytes as initiators of inflammation. Lancet 337:211–214
Baus E, Andris F, Dubois PM, Urbain J, Leo O (1996) Dexamethasone inhibits the early steps of antigen receptor signaling in activated T lymphocytes. J Immunol 156:4555–4561
Bekersky I, Fitzsimmons W, Tanase A, Maher RM, Hodosh E, Lawrence I (2001) Nonclinical and early clinical development of tacrolimus ointment for the treatment of atopic dermatitis. J Am Acad Dermatol 44:S17–S27
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771
Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H (1999) Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol 103:119–124
Dustin ML, Singer KH, Tuck DT, Springer TA (1988) Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med 167:1323–1340
Gavrieli Y, Sherman Y, Ben Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Grabbe S, Schwarz T (1998) Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today 19:37–44
Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J (1998) A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today 19:359–361
Grewe M, Gyufko K, Schopf E, Krutmann J (1994) Lesional expression of interferon-gamma in atopic eczema. Lancet 343:25–26
Grewe M, Vogelsang K, Ruzicka T, Stege H, Krutmann J (2000) Neurotrophin-4 production by human epidermal keratinocytes: increased expression in atopic dermatitis. J Invest Dermatol 114:1108–1112
Griffiths CE, Voorhees JJ, Nickoloff BJ (1989) Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol 20:617–629
Hanifin JM, Tofte SJ (1999) Update on therapy of atopic dermatitis. J Allergy Clin Immunol 104:S123-S125
Herz U, Bunikowski R, Renz H (1998) Role of T cells in atopic dermatitis. New aspects on the dynamics of cytokine production and the contribution of bacterial superantigens. Int Arch Allergy Immunol 115:179–190
Ibanez CF, Ernfors P, Timmusk T, Ip NY, Arenas E, Yancopoulos GD, Persson H (1993) Neurotrophin-4 is a target-derived neurotrophic factor for neurons of the trigeminal ganglion. Development 117:1345–1353
Kay JE, Benzie CR, Goodier MR, Wick CJ, Doe SE (1989) Inhibition of T-lymphocyte activation by the immunosuppressive drug FK-506. Immunology 67:473–477
Kim DS, Kim HJ, Choi KH, Chung JH, Kim KH, Par KC (2001) UVB-induced GM-CSF production is suppressed by dexamethasone in HaCaT cells. Photodermatol Photoimmunol Photomed 17:121–125
Klunker S, Trautmann A, Akdis M, Verhagen J, Schmid-Grendelmeier P, Blaser K, Akdis CA (2003) A second step of chemotaxis after transendothelial migration: keratinocytes undergoing apoptosis release IFN-gamma-inducible protein 10, monokine induced by IFN-gamma, and IFN-gamma-inducible alpha-chemoattractant for T cell chemotaxis toward epidermis in atopic dermatitis. J Immunol 171:1078–1084
Krueger JG, Krane JF, Carter DM, Gottlieb AB (1990) Role of growth factors, cytokines, and their receptors in the pathogenesis of psoriasis. J Invest Dermatol 94:135S–140S
Lange K, Kleuser B, Gysler A, Bader M, Maia C, Scheidereit C, Korting HC, Schafer-Korting M (2000) Cutaneous inflammation and proliferation in vitro: differential effects and mode of action of topical glucocorticoids. Skin Pharmacol Appl Skin Physiol 13:93–103
Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K (1989) The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science 243:1464–1466
Laughter D, Istvan JA, Tofte SJ, Hanifin JM (2000) The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol 43:649–655
Leung DY (2000) Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol 105:860–876
Lever R, MacDonald C, Waugh P, Aitchison T (1998) Randomised controlled trial of advice on an egg exclusion diet in young children with atopic eczema and sensitivity to eggs. Pediatr Allergy Immunol 9:13–19
Levin C, Maibach HI (2000) An overview of the efficacy of topical corticosteroids in experimental human nickel contact dermatitis. Contact Dermatitis 43:317–321
Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda EA, Rothenberg ME, Drazen JM, Luster AD (1997) Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest 99:1767–1773
Meyle J, Gultig K, Rascher G, Wolburg H (1999) Transepithelial electrical resistance and tight junctions of human gingival keratinocytes. J Periodontal Res 34:214–222
Michel G, Auer H, Kemeny L, Bocking A, Ruzicka T (1996) Antioncogene P53 and mitogenic cytokine interleukin-8 aberrantly expressed in psoriatic skin are inversely regulated by the antipsoriatic drug tacrolimus (FK506). Biochem Pharmacol 51:1315–1320
Mochizuki M, Bartels J, Mallet AI, Christophers E, Schröder JM (1998) IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol 160:60–68
Mukaida N, Gussella GL, Kasahara T, Ko Y, Zachariae CO, Kawai T, Matsushima K (1992) Molecular analysis of the inhibition of interleukin-8 production by dexamethasone in a human fibrosarcoma cell line. Immunology 75:674–679
Nakagawa H, Etoh T, Ishibashi Y, Higaki Y, Kawashima M, Torii H, Harada S (1994) Tacrolimus ointment for atopic dermatitis. Lancet 344:883
Nickoloff BJ, Griffiths CE, Barker JN (1990) The role of adhesion molecules, chemotactic factors, and cytokines in inflammatory and neoplastic skin disease—1990 update. J Invest Dermatol 94:151S–157S
Ollmar S, Eek A, Sundstrom F, Emtestam L (1995) Electrical impedance for estimation of irritation in oral mucosa and skin. Med Prog Technol 21:29–37
Riddle DR, Lo DC, Katz LC (1995) NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature 378:189–191
Rowland Payne CM, Wilkinson JD, McKee PH, Jurecka W, Black MM (1985) Nodular prurigo—a clinicopathological study of 46 patients. Br J Dermatol 113:431–439
Santamaria Babi LF, Picker LJ, Perez Soler MT, Drzimalla K, Flohr P, Blaser K, Hauser C (1995) Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med 181:1935–1940
Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM (1999) Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol 104:1273–1279
Schröder JM, Noso N, Sticherling M, Christophers E (1996) Role of eosinophil-chemotactic C-C chemokines in cutaneous inflammation. J Leukoc Biol 59:1–5
Slavin J, Unemori E, Hunt TK, Amento E (1995) Monocyte chemotactic protein-1 (MCP-1) mRNA is down-regulated in human dermal fibroblasts by dexamethasone: differential regulation by TGF-beta. Growth Factors 12:151–157
Springer M, Engelhart K, Biesalski HK (2003) Effects of 3-isobutyl-1-methylxanthine and kojic acid on cocultures and skin equivalents composed of HaCaT cells and human melanocytes. Arch Dermatol Res 295:88–91
Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Brocker EB, Blaser K, Akdis CA (2000) T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 106:25–35
Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Brocker EB, Blaser K, Akdis CA (2001a) Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol 108:839–846
Trautmann A, Altznauer F, Akdis M, Simon HU, Disch R, Brocker EB, Blaser K, Akdis CA (2001b) The differential fate of cadherins during T-cell-induced keratinocyte apoptosis leads to spongiosis in eczematous dermatitis. J Invest Dermatol 117:927–934
Tuschil A, Lam C, Haslberger A, Lindley I (1992) Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. J Invest Dermatol 99:294–298
Van Snick J (1990) Interleukin-6: an overview. Annu Rev Immunol 8:253–278
Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG (2000) A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol 115:640–646
Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I (2004) Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol 150:910–916
Wakugawa M, Nakamura K, Akatsuka M, Nakagawa H, Tamaki K (2001) Interferon-gamma-induced RANTES production by human keratinocytes is enhanced by IL-1beta, TNF-alpha, IL-4 and IL-13 and is inhibited by dexamethasone and tacrolimus. Dermatology 202:239–245
Wen LP, Madani K, Fahrni JA, Duncan SR, Rosen GD (1997) Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol 273:L921-L929
White MV, Yoshimura T, Hook W, Kaliner MA, Leonard EJ (1989) Neutrophil attractant/activation protein-1 (NAP-1) causes human basophil histamine release. Immunol Lett 22:151–154
Yang Y, Mercep M, Ware CF, Ashwell JD (1995) Fas and activation-induced Fas ligand mediate apoptosis of T cell hybridomas: inhibition of Fas ligand expression by retinoic acid and glucocorticoids. J Exp Med 181:1673–1682
Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ (1987) Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA 84:9233–9237
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engelhart, K., El Hindi, T., Biesalski, HK. et al. In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Arch Dermatol Res 297, 1–9 (2005). https://doi.org/10.1007/s00403-005-0575-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-005-0575-7