Abstract
There is a growing evidence that cytokines are important in the depigmentation process of vitiligo, however, the exact mechanism is not fully understood. The aim of this work was to study the possible role of the tumor necrosis factor-α (TNF-α) cytokine in the depigmentation process of the disease. Twenty patients with generalized vitiligo were exposed to narrow-band ultraviolet B (NB-UVB) therapy thrice weekly for a total of 60 sessions. Immunohistochemical examination was done, to assess the TNF-α expression in lesional and perilesional skin as compared to normal control skin, before and after therapy. At baseline, positive lesional TNF-α expression was detected in 60 % of patients which was significantly higher as compared to perilesional skin (20 %) and negative expression in healthy control skin. Post-treatment, a statistically significant increase in TNF-α expression was detected in both lesional (90 %) and perilesional skin (70 %) as compared to baseline (P < 0.05). The significant increase of TNF-α in vitiligo lesions compared with perilesional and healthy skin suggests a possible involvement of this cytokine in the depigmentation of vitiligo. The increase in TNF-α expression after NB-UVB phototherapy suggests another role in repigmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitiligo is an idiopathic, acquired depigmenting disorder characterized by loss of functional melanocytes from the epidermis. Several types of vitiligo are distinguished according to the distribution of achromic lesions. The generalized type is characterized by multiple scattered lesions in a more or less symmetrical distribution pattern [29]. The course of the disease is unpredictable, but is often progressive with phases of stabilized depigmentation [6].
There is a growing evidence that keratinocyte-derived cytokines such as TNF-α are important in the depigmentation process of vitiligo, however, the exact mechanism is not fully understood [12, 19]. Keratinocytes secrete tumor necrosis factor-α (TNF-α), interleukin-1α (IL-Iα) and transforming growth factor-β (TGF-β), which are potent inhibitors of melanocyte proliferation and melanogenesis, and also secrete granulocyte macrophage colony-stimulating factor (GM-CSF), endothelins and basic fibroblast growth factor (b-FGF) which are mitogens for melanocytes [2].
The mechanism of action of narrow-band ultraviolet B (NB-UVB) phototherapy in vitiligo is not completely understood. It probably involves release of cytokines and inflammatory mediators in the skin that stimulate melanocyte migration and proliferation [22]. UVB radiation has been shown to be a potent inducer of TNF-α gene expression which mediates signaling by human keratinocytes [11].
The aim of this work was to study the possible role of the TNF-α cytokine in the pathogenesis of vitiligo.
Patients and methods
Twenty patients with generalized symmetric vitiligo were included in this study: 14 females and six males ranging in age from 12 and 52 years with a mean of 25.3 years. Criteria for exclusion included pregnancy, lactation, phototherapy within past 6 months, history of cutaneous photosensitivity, eye cataract or skin cancer and patients on immunosuppressive therapy. The research protocol was approved by the local ethics committee and all subjects provided written informed consent.
All patients were subjected to complete dermatological examination with evaluation of extent of involvement using the “hand palm rule” (size of physician’s hand palm equals 1 % of the total body area) [10].
Affected segments of the patients were exposed to NB-UVB therapy thrice weekly on non-consecutive days for a total of 60 sessions using the phototherapy unit GH-8ST (Cosmedico Medizintechnik, Schwenningen, Germany) with a spectrum of 305–315 nm and a maximum wavelength of 311 nm providing an irradiance of 6–8 mW/cm2. The starting dose was chosen according to skin phototype using ready calibrated table supplied by the manufacturer: 0.411 J/cm2 for patients with skin type III, 0.574 J/cm2 for patients with skin type IV, and 0.707 J/cm2 for patients with skin type V.
The clinical response to therapy was visually scored as the percentage of repigmentation of depigmented lesions at the end of therapy [8] and recorded as excellent response (>75 %), good response (51–75 %), moderate response (26–50 %), poor response (<25 %) and no response (0) depending on the extent of repigmentation for any specific lesion. Color photographs were performed as a baseline and after 20 sessions and at the end of therapy (60 sessions). Treated areas were evaluated at each subsequent visit for side effects of therapy such as erythema, pruritus and burning sensation.
Five-millimeter punch biopsy specimens were obtained both before and after therapy from the lesional and perilesional unexposed skin of each patient and from the exposed skin of the face of two patients. The control skin biopsies were obtained from uninvolved remote areas of each patient and from ten healthy normal volunteers (matched for age and sex). The specimens were divided into two groups, the first group was fixed in 10 % formalin solution and embedded in paraffin to form paraffin blocks. Serial sections of 4 μ thickness were obtained from each block and subjected to hematoxylin and eosin stain for histologic diagnosis. Identification of melanocytes was performed by streptavidin–biotin method using primary antibodies against gp100 (HMB45, DAKO) [28]. Cytoplasmic staining alone was considered positive for HMB45.
Immunohistochemical staining using streptavidin–biotin immuno-peroxidase technique (Strept A-B staining method) was performed for the detection of TNF-α expression, using an anti-human TNF-α/TNFSF1A antibody (R&D systems, Inc. USA) [28]. TNF-α was expressed in the form of brown cytoplasmic precipitate. The percentage of positive cells was calculated in 100 cells counted in 4 high power fields.
Criteria for grading stained sections were:
-
Negative (−): if <5 % of cells stained.
-
Weakly positive (+): if 5–25 % of cells stained.
-
Moderately positive (++): if 26–50 % of cells stained.
-
Strongly positive (+++): if >50 % of cells stained.
The second group of the biopsy specimens were frozen in liquid nitrogen (−196 °C) and preserved at −70 °C. Levels of TNF-α were measured with specific ELISA kits (Biosource International, Camarillo, CA, USA) according to the manufacturer’s instructions. TNF-α was measured as described by Lowry et al. [17]. Level of the cytokine was expressed as μmol/mg protein.
Statistical analysis
The data were tabulated and statistically analyzed using Epi-info 2005 and SPSS version 10 software package. The qualitative data were evaluated using chi-squared (χ2) and Mc Nemar’s test for paired analysis when appropriate. The significance level was considered at P value <0.05.
Results
Evaluation of the clinical response of patients to NB-UVB therapy showed that three patients (15 %) had excellent response, five (25 %) had good response, six (30 %) had moderate response and six (30 %) had poor response with a total cumulative dose of 218.3 ± 9.5 J/cm2 (range 198.375–226.721 J/cm2) at the end of therapy. The best results were recorded in facial lesions while hands and feet lesions were less responsive to therapy.
Excellent response was detected in 30 % of facial lesions, 14.3 % of lower extremities lesions, 11.8 % of upper extremities lesions and 7.7 % of trunk lesions with no statistically significant difference between response in these sites (P > 0.05) (Figs. 1, 2).
The side effects observed during therapy were mild and well tolerated. They were reported in five patients (25 %); three of them reported mild erythema and two patients complained of pruritus.
Histopathological examination of vitiligo lesions before treatment revealed that there was acanthosis in 40 % of cases. The most important finding was the complete disappearance of melanocytes in 80 % of cases while very few melanocytes with pigmentation in basal cell layer were still detected in the remaining 20 % of cases. The dermis was infiltrated with mononuclear inflammatory cells.
After treatment, histopathological examination revealed normal thickness of the epidermis in 30 % of cases, thinning of the epidermis was reported in 20 % of cases, while the remaining of cases showed acanthosis. There was an increased density of melanin pigmentation and reappearance of melanocytes. The dermis contained a perivascular lymphocytic inflammatory infiltrate as well as scattered melanophages.
Immunostaining with HMB45 of the control group skin biopsies showed strongly staining melanocytes in the basal layer of the epidermis, while no positively staining melanocytes were detected in any lesional skin before therapy. After treatment, the appearance of melanocytes in the epidermis of biopsies of vitiliginous skin was demonstrated by positive staining for HMB45, though with different staining intensity from the control biopsies.
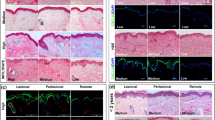
Immunohistochemical examination of the control group skin biopsies revealed lack of expression of TNF-α in all specimens (Fig. 3). In contrast, vitiliginous skin revealed positive expression in 60 % of patients and that TNF-α staining was diffuse in the specimens and extended throughout all epidermal layers. Most of the dermal cell infiltrate was TNF-α positive. Other structures within the dermis like endothelial cells, eccrine glands, hair follicles and even the erector pilli muscles revealed different degrees of positivity to TNF-α. In these structures, TNF-α expression was detected in all cells after treatment compared to ≤50 % of the cells before treatment (Figs. 4, 5).
a Immunohistochemical picture of exposed skin lesion before NB-UVB phototherapy showing mild positive staining reaction of the epidermis for TNF-α (DAB ×200). b Immunohistochemical picture of the same skin lesion after NB-UVB phototherapy showing strong positive staining reaction of the epidermis for TNF-α with scattered positive inflammatory cells, eccrine glands and hair follicles (DAB ×100)
a Immunohistochemical picture of the unexposed skin lesion before NB-UVB therapy showing moderate cytoplasmic (arrow) staining (++) for TNF-α throughout all epidermal layers (DAB ×400). b Immunohistochemical pictures of the same lesion after NB-UVB therapy showing strong cytoplasmic (arrow) staining (+++) for TNF-α throughout all epidermal layers (DAB ×400)
Immunohistochemical examination before therapy showed a statistically significant difference in TNF-α expression between lesional and perilesional skin (P < 0.05). Positive TNF-α expression was detected in 60 % of patients in lesional skin compared to 20 % in perilesional skin. A statistically significant difference was also detected between the staining grade of TNF-α in lesional skin as compared to perilesional skin (P < 0.05). In lesional skin, the positive staining was equally distributed among weak, moderate and strong intensities (12 patients total), whereas only weak staining was seen in perilesional skin (Tables 1, 2).
Immunohistochemical examination of vitiligo lesions after treatment revealed diffuse TNF-α expression in lesional skin (90 % of cells stained) compared to 70 % in perilesional skin, however, the difference was statistically insignificant (P > 0.05). Comparison of TNF-α expression in the lesional skin before and after NB-UVB therapy showed a statistically significant increased expression from 60 % before treatment to 90 % post-treatment (P < 0.05). A significant increase in the TNF-α expression in perilesional skin was also noted from 20 % before treatment to 70 % after therapy (P < 0.05) (Tables 1, 2).
No statistically significant association was detected between the staining intensity and the excellent, good and moderate lesional clinical response of the unexposed and the exposed skin (P > 0.05). A highly statistically significant association was detected between negative staining reaction and no response of lesions to therapy (P = 0.0001). On the other hand, in lesions with poor response (5 lesions), 60 % (3 lesions) showed weakly positive staining reaction and 40 % (2 lesions) showed moderate positivity which was statistically significant (P < 0.05) (Table 3).
Using ELISA technique, the level of TNF-α was significantly higher in lesional skin compared with the perilesional skin before and after NB-UVB therapy (P < 0.001) (Table 4).
Discussion
Treatment of vitiligo is still a challenge for dermatologists. Despite different therapeutic approaches, vitiligo remains a difficult disease to treat without a definite cure [1]. NB-UVB has recently emerged as a promising mode of therapy for generalized vitiligo. The detailed mechanism of action of NB-UVB is not yet fully understood but involves a combination of effects in cell cycle kinetics, alterations in cytokine expression, effect on melanocytes and immunomodulation [26].
A two-part process has been proposed as an explanation of the mechanism of NB-UVB induced repigmentation, where both processes may occur simultaneously. It involves the stabilization of the depigmentation process and the stimulation of residual follicular melanocytes [7]. In particular, NB-UVB radiation probably stimulates the upregulation of the melanogenesis and melanocyte migration [14].
The best clinical results on face and neck were explained that NB-UVB stimulates the melanocytic reservoirs in the hair sheaths as repigmentation occurred in a perifollicular pattern and was not observed in lesions with white amelanotic hair [20]. Brazzelli et al. [3] added that the face, in particular, is a body site with a great number of pilosebaceous units which are activated by NB-UVB. The amelanotic melanocytes in the outer hair root sheaths proliferate and migrate resulting in perifollicular repigmentation. The lower repigmentation rates of acral regions may be attributed to lack of hair follicles in acral areas. The histopathological examination corresponds to the clinical response, while no statistically significant association was detected between the staining intensity and the excellent, good and moderate lesional clinical response of the unexposed and the exposed skin.
In our study, pigmentation was observed more within the infundibula of hair follicles. This observation concurs with a previous report by Cui et al. [5].
Melanocytic mitogenesis, melanogenesis, and migration have been shown to be induced by various cytokines and inflammatory mediators, including IL-1, TNF-α and leukotriene 4 [21]. IL-1α stimulates the synthesis of endothelin-1, a potent vasoconstrictive peptide that has mitogenic and melanogenic properties [23]. UVB radiation has been shown to be a potent inducer of TNF-α gene expression which mediates signaling by human keratinocytes. It is suggested that NB-UVB alters the production of cytokines and chemokines as a combined result of its direct and indirect TNF-α-mediated effects [11].
On the other hand, susceptibility to UVB-induced immunosuppression is partly controlled by the TNF locus-α which is involved in Langerhans cell migration from the skin; induction of this cytokine could lead to altered, or diminished antigen presentation via Langerhans cell depletion [24].
Immunohistochemical examination of vitiligo lesions and perilesional skin before therapy showed a statistically significant difference in TNF-α expression. Positive expression was detected in 60 % of patients in lesional skin compared to 20 % in perilesional skin.
Our results were in agreement with those of Moretti et al. [19] and Birol et al. [2], concerning the higher TNF-α expression in vitiligo lesional skin compared to perilesional skin, non-lesional skin and healthy skin. These findings were explained by the possibility of the in situ inhibition of melanogenesis induced by TNF-α throughout an inhibitor loop on tyrosinase and TRP-1 [19].
An increase in TNF-α expression was detected in vitiligo lesions after NB-UVB therapy. We did not expect this result, and so we tried to find an explanation to that increase after therapy. To the best of our knowledge, this is the first immunohistochemical study to evaluate TNF-α expression after NB-UVB treatment.
In contrast, Grimes et al. [9] using quantitative PCR technique reported reduced expression of TNF-α after topical tacrolimus therapy, suggesting a possible role of TNF-α in the depigmentation process. This difference in results could be due to the different techniques used or due to the different modes of therapy and their actions in inducing repigmentation such as the immunomodulatory effects on T cells and other immunocompetent cells and the production of cytokines and chemokines.
On the other hand, other studies have detected an increase in TNF-α expression after UVB treatment [4, 15, 16]. They reported that UVB irradiation appeared to be a significant inducer of TNF-α transcription in epidermal cells in vitro and in vivo. Skov et al. [27] found that irradiating human skin with three MED of UVB led to rapid increase in TNF-α protein in suction blister fluid, which was maximal at 6 h following exposure. Similarly, Hino et al. [11] investigated the effect of NB-UVB on the production of chemokines and proinflammatory cytokines by keratinocytes in comparison with BB-UVB. They found that TNF-α m RNA levels were immediately up regulated after exposure of the spontaneously transformed human epidermal cell line HaCaT to UVB radiation and confirmed the results of increased production of TNF-α after UVB irradiation.
The mechanism of how TNF affects pigmentation is not fully understood. It was suggested that cytokines such as TNF-α, TNF-β, INF-γ, IL-Iα, and IL-6 can induce cell surface ICAM-1 on melanocytes which is necessary for leukocyte–melanocyte attachment. ICAM-1 may also induce B-cell activation, increasing autoantibody production and may cause melanocyte damage in vitiligo [18]. TNF-α and IL-Iα also have the capacity to induce apoptosis in many cell types [2].
The proapoptotic potential of TNF-α has been demonstrated for various cell types, whereas nuclear factor-kappaB (NF-kappaB) is known to support the transcription of prosurvival genes. In a previous study, investigation of normal human melanocytes revealed induction of apoptosis after TNF-α treatment (100 U/ml) in only 3 out of 11 cultures analyzed, whereas 8 cultures remained largely resistant. In sensitive cultures, NF-kappaB binding activity was found increased after TNF-α treatment; apoptosis-resistant cells were characterized by relatively high basic NF-kappaB binding activities and did not show NF-kappaB activation after TNF-α treatment. Inhibition of NF-kappaB by a specific inhibitor, Bay-11, either induced apoptosis itself or resistant melanocyte cultures became sensitive to TNF-α treatment. No correlation was found between apoptosis sensitivity and the expression of TNF receptor-1 or the expression of Bax, Bcl-2 and Bcl-X (L). A strong correlation, however, was found regarding the pigmentation degree, as high pigmentation correlated with apoptosis resistance and sensitive melanocyte cultures were weakly pigmented. These data may indicate that in cultured melanocytes, high levels of melanogenesis lead to an increase in oxidative stress which itself causes NF-kappaB activation. NF-kappaB mediates the transcription of antiapoptotic factors which may block TNF-α-induced apoptosis at early steps of the signal cascade [25].
Some authors studied whether TNF-α and interleukin IL1α, which may be implicated in the inflammatory phenomena seen in acute GVHD, are implicated in the pigmentary changes or not. The expression of TNF-α increased in the hyperpigmented skin relative to normal and hypopigmented skin and TNF-α was variably distributed in proportion to different degrees of pigmentation. This observation may indicate that the production of TNF-α by epidermal microenvironment may be involved in postinflammatory pigmentary changes [13].
It was surprising to find an increase in TNF-α expression in vitiligo lesions after NB-UVB therapy which was confirmed using the quantitative ELISA technique. We tried to find an explanation for this observation as this is the first immunohistochemical study for the detection of TNF-α expression in vitiligo lesions after NB-UVB therapy. The increase in TNF-α expression in the treated lesions (pigmented) in comparison to lesional skin (hypopigmented) and normal skin predicts that the role of TNF-α in pigmentation may be dose-dependent. So, further studies are needed to detect to which level there is inhibition of pigmentation and another for repigmentation.
Clinically, IL-1α and TNF-α induce cutaneous inflammation. The production of TNF-α by epidermal microenvironment may be also involved in post-inflammatory pigmentary changes.
Susceptibility to UVB-induced immunosuppression is partly controlled by the TNF locus-α involved in Langerhans cell migration from the skin, and induction of this cytokine could lead to altered, or diminished, antigen presentation, via Langerhans cell depletion [24], which may decrease antibodies production, preventing melanocyte damage in vitiligo.
The role of TNF-α in repigmentation may be also explained by a previous study [25], which reported that an increase in oxidative stress secondary to the high levels of NB-UVB-induced melanogenesis may itself cause NF-kappaB activation which block TNF-α-induced apoptosis at early steps of the signal cascade, so melanocytes resist apoptosis.
In conclusion, this study demonstrated a significant increase of TNF-α in vitiligo lesions, compared with perilesional and healthy skin, thus suggesting a possible involvement of this cytokine in the pathogenesis of vitiligo. After NB-UVB therapy, the increase of TNF-α in vitiligo lesions suggests another role for repigmentation in vitiligo. Further studies using different methods of investigations and different remedies of therapy are needed to elucidate the possible role of the TNF-α cytokine and other cytokines in the depigmentation and repigmentation processes in vitiligo.
References
Arca E, Tastan HB, Erbil AH et al (2006) Narrow band ultraviolet B as monotherapy and in combination with topical calcipotriol in the treatment of vitiligo. J Dermatol 33:338–343
Birol A, Kisa U, Kurtipek GS et al (2006) Increased tumour necrosis factor alpha (TNF-α) and interleukin 1 alpha (IL-1α) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol 45:992–993
Brazzelli V, Antoninetti M, Palazzini S et al (2007) Critical evaluation of the variants influencing the clinical response of vitiligo: study of 60 cases treated with NB-UVB phototherapy. J Eur Acad Dermatol Venereol 21:1369–1374
Brink N, Szamel M, Young AR et al (2000) Comparative quantification of IL-1β, IL-10, TNF-α and IL-7 mRNA levels in UV irradiated skin in vivo. Inflamm Res 49:290–296
Cui J, Shen LY, Wang GC (1991) Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol 97:410–416
Eves PC, Bullett NA, Haddow D et al (2008) Simplifying the delivery of melanocytes and keratinocytes for the treatment of vitiligo using a chemically defined carrier dressing. J Invest Dermatol 128(6):1554–1564
Fitzpatrick TB (1997) Mechanisms of phototherapy in vitiligo. Arch Dermatol 133:1591–1592
Goktas EO, Aydin F, Senturk N et al (2006) Combination of narrow band UVB and topical calcipotriol for the treatment of vitiligo. J Eur Acad Dermatol Venereol 20:553–557
Grimes PE, Morris R, Avaniss-Aghajani T et al (2004) Topical tacrolimus therapy for vitiligo. Therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol 51(1):52–61
Hamzavi I, Jain H, McLean D et al (2004) Parametric modeling of narrow band UV-B phototherapy for vitiligo using a novel quantitative tool. Arch Dermatol 140:677–683
Hino R, Kobayashi T, Mori H et al (2007) Inhibition of T helper 2 chemokine production by narrow-band ultraviolet B in cultured keratinocytes. Br J Dermatol 156:830–837
Joshi PG, Nair N, Begum G et al (2007) Melanocyte-keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigment Cell Res 20(5):380–384
Kang HY, Kang WH (2004) Leukomelanoderma following acute cutaneous graft-versus-host disease. Eur J Dermatol 14(3):146–149
Kawaguchi M, Mitsuhashi Y, Kondo S (2005) Over expression of tumor necrosis factor α-converting enzyme in psoriasis. Br J Dermatol 152(5):915–919
Köck A, Shwarz T, Kirnbauer R et al (1990) Human keratinocytes are a source for tumour necrosis factor α: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 172:1609–1614
Leverkus M, Yaar M, Eller MS et al (1998) Post transcriptional regulation of UV induced TNF-alpha expression. J Invest Dermatol 110:353–357
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Martinez-Esparzo M, Jimenez-Cervantes C, Solano F et al (1998) Mechanisms of melanogenesis inhibition by tumour necrosis factor-α in melanoma cells. Eur J Biochem 255:139–146
Moretti S, Spallanzani A, Amato L et al (2002) New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res 15:87–92
Njoo MD, Bos JD, Westerhof W (2000) Treatment of generalized vitiligo in children with narrow band (TL-01) UVB radiation therapy. J Am Acad Dermatol 42:245–253
Scherschun L, Kim JJ, Lim HW (2001) Narrow band ultraviolet-B is a useful and well tolerated treatment for vitiligo. J Am Acad Dermatol 44(6):999–1003
Schwarz A, Maeda A, Wild MK et al (2004) Ultraviolet radiation induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol 172:1036–1043
Schwarz T (2002) Photoimmunosuppression. Photodermatol Photoimmunol Photomed 18:141–145
Seité S, Colige A, Deroanne C et al (2004) Changes in matrix gene and protein expressions after single or repeated exposure to one minimal erythemal dose of solar-stimulated radiation in human skin in vivo. Photochem Photobiol 79:265–271
Shang J, Eberle J, Geilen CC et al (2002) The role of nuclear factor-kappa B and melanogenesis in tumor necrosis factor-alpha-induce. Skin Pharmacol Appl Skin Physiol 15:321–329
Shintani Y, Yasuda Y, Kobayashi K et al (2008) Narrow band UVB radiation suppresses contact hypersensitivity. Photodermatol Photoimmunol Photomed 24:32–37
Skov L, Hansen H, Allen M et al (1998) Contrasting effects of ultraviolet A1 and ultraviolet B exposure on the induction of tumour necrosis factor alpha in human skin. Br J Dermatol 138:216–220
Wallace ML, Smoller BR (1996) Immunohistochemistry in diagnostic dermatopathology. J Am Acad Dermatol 34(2):163–183
Westerhof W, d’ Ischia M (2007) Vitiligo puzzle, the pieces fall in place. Pigment Cell Res 20(5):345–359
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attwa, E., Gamil, H., Assaf, M. et al. Over-expression of tumor necrosis factor-α in vitiligo lesions after narrow-band UVB therapy: an immunohistochemical study. Arch Dermatol Res 304, 823–830 (2012). https://doi.org/10.1007/s00403-012-1269-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-012-1269-6