Abstract
Acne is a multifactorial, chronic inflammatory disease of pilosebaceous unit in which cytokines have been implicated in the pathogenesis. Although it is thought to be an inherited disease, there are limited data supporting the relevant genetic elements. Tumor necrosis factor-alpha (TNF-alpha) is one of the proinflammatory cytokines involved in the acne pathogenesis. Several single-nucleotide polymorphisms (SNPs) have been identified in the human TNF-alpha gene promoter. The polymorphism at position -308, which involves substituting guanine (G) for adenine (A) (TNFA-308 G/A) has been linked to increased susceptibility to several chronic inflammatory diseases. The aim of this study was to determine the TNFA-308 G/A polymorphism in acne and to examine whether there is a relationship between this polymorphism and disease susceptibility. Exactly, 113 patients with acne and 114 healthy control subjects were included in the study. Polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) assay was used for analysis of the TNFA-308 G/A polymorphism. We found that the frequency of the TNFA-308 GA genotype was statistically significantly increased in patients compared with healthy controls (P < 0.001). There was no association between TNFA genotypes and severity of acne (P > 0.05). There was also no significant difference between male and female patients. Our results suggest that TNFA-308 G/A polymorphism may contribute to a predisposition to acne in Turkish population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acne is a chronic inflammatory disease of pilosebaceous unit widely affecting adolescents and young adults [19]. It is a multifactorial disease and several pathogenetic factors have been identified including sebum overproduction, abnormal follicular keratinization, Propionibacterium acnes (P. acnes) proliferation, inflammation, delayed-type immune response, external factors, and genetics [17, 19, 23, 30, 46]. The genetic influence on the pathogenesis of the disease has been well documented in twins [9, 17, 18, 42], but there are only few studies investigating the relevant genetic elements [5, 22, 29, 34, 35, 39].
It is suggested that acne vulgaris is likely to be a genuine inflammatory disease [30, 46]. Tumor necrosis factor-alpha (TNF-alpha) is one of the main proinflammatory cytokines that play a central role in initiating and regulating the cytokine cascade during an inflammatory response [8]. It has an important role in the pathogenesis of acne as in the other inflammatory skin diseases [21, 30, 41]. Factors affecting its production may possibly influence the degree of inflammatory response and hence may account for the clinical severity of acne. The TNF-alpha gene is located on chromosome 6 (6p21.3) between HLA-B and DR within the class III region of the major histocompatibility complex [13]. Several single-nucleotide polymorphisms (SNPs) in the TNF gene promoter have been identified, some of which may regulate TNF-alpha expression. One of them represents a guanine (G) to adenine (A) transition at position -308 (TNFA-308 G/A), and has been examined in several inflammatory diseases [6, 7, 14, 15, 28, 40, 44]. The aim of our study was to investigate for the first time whether TNFA-308 G/A polymorphism might be involved in the pathogenesis of acne and whether there is a relationship between this polymorphism and severity of the disease.
Materials and methods
Subjects
We examined 113 patients with acne (90 women, 23 men) and 114 healthy control subjects (45 men, 69 women). The diagnosis of acne was based on a thorough physical examination. The clinical grade of acne was assessed based on the Global Acne Grading System [16]. According to the system, patients were divided into four categories namely mild, moderate, severe, and very severe acne. The control group was chosen from healthy individuals without any systemic and dermatologic disease. The study was approved by the local ethic committee and informed consent was obtained from each individual before sample collection.
Analysis of TNFA -308 G/A polymorphism
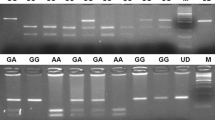
Venous blood samples were collected in ethylenodiaminotetra acetic acid (EDTA) containing tubes. DNA was extracted from whole blood using a genomic DNA purification kit (MBI Fermentas, Vilnius, Lithuania). Polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) assay was used to determine TNFA -308 G/A polymorphism. The oligonucleotide primers used to determine the -308 G/A polymorphism within the TNF-alpha gene that were described previously [10]. The primers, forward 5′-AGGCAATAGGTTTTGAGGGCCAT-3′,s reverse 5′-TCCTCCCTGCTCCGATTCCG-3′ were used to amplify the TNF-alpha gene. PCR was performed in a 25 μl volume with 100 ng DNA, 100 μm dNTPs, 20 pmol of each primer, 1.5 mM MgCl2, 1x PCR buffer with (NH4)SO4 (MBI Fermentas, Vilnius, Lithuania,), 10% DMSO, and 2U Taq DNA polymerase (MBI Fermentas, Vilnius, Lithuania). Amplification was performed on an automated thermal cycler (Techne Flexigene, Cambridge, UK). PCR conditions were 2 min for initial denaturation at 95°C; 35 cycles at 95°C for 45 s for denaturation, 1 min at 60°C for annealing and 90 s at 72°C for extension, followed by 7 min at 72°C for final extension. The PCR products were 107 bp. After amplification, PCR products were digested by restriction endonuclease 10U NcoI (MBI Fermentas, Vilnius, Lithuania) for 14 h at 37°C. The genotyping of the TNF-alpha gene was determined by fragment separation at 120 V for 40–50 min on a 3.5% agarose gel containing 0.5 μg/ml ethidium bromide. A 50 bp marker (50 bp DNA Ladder, MBI Fermentas) was used as a size standard for each gel lane. The gel was visualized under UV light using a gel electrophoresis visualizing system (Vilber Lourmat). The NcoI restricted products of TNFA-308 G/A; GG, GA, and AA genotypes had band sizes of 87bp/20 bp, 107bp/87bp/20 bp, and 107 bp, respectively. Selected samples from each gel were repeated to confirm the results. Genotyping was based upon independent scoring of the results by two reviewers who were unaware of case/control status.
Statistical analysis
All statistical tests were carried out using SPSS 11.5 for Windows. The distribution of genotypes and Hardy–Weinberg equilibrium were tested with Chi-square (χ2) test for quality of fit. The z-approximation test was used to compare two independent proportions of patients and control subjects. P values smaller than 0.05 were considered statistically significant. The strength of association was estimated by calculating the odds ratios (ORs) and 95% confidence intervals (95% CIs) from the 2 × 2 table data.
Results
Of the 113 patients, 32 suffered from mild acne whereas 51 had moderate, and 30 had severe acne. The main age was 30.6 ± 13.2 for the control subjects and 22.0 ± 7.4 for the patients.
All patients and control subjects were genotyped at the TNFA-308 locus. The distribution of genotypes was in Hardy–Weinberg equilibrium in all groups. Genotype frequencies for the patients and controls are presented in Table 1. The distribution of the GG, GA, and AA genotypes was 58.4, 38.1, and 3.5%, respectively, in acne patients. Genotypes in healthy controls were as follows: GG 86.8%, GA 13.2%, and AA 0%. The difference in the distributions of the TNFA genotypes between the patient and control group was statistically significant. The GA genotype frequency was significantly increased in acne patients (P < 0.001, OR 4.054, 95% CI 2.090–7.865) compared with healthy controls.
The severity of acne was not associated with TNFA genotypes (P = 0.463) as shown in Table 2. GG, GA, and AA genotype distributions were similar between male and female patients (P = 0.518). There was also no male/female difference in the distribution of GG and GA genotypes (P = 0.602) in the controls (Table 3).
Table 4 shows a comparison of the distribution for TNFA genotypes of our control subjects with those of other populations from Turkey and different countries. When the genotype frequencies of our controls were compared with those of other populations from Turkey [6, 7, 26], Iran [25], and Korea [28], there were no significant differences. However, when the genotype frequencies of our controls were compared to those of European people from Italy [32], and England [37], genotype distributions differed; GA genotype frequency was found to be decreased in our controls, while GG genotype frequency was found to be decreased in European populations (P < 0.001).
Discussion
During the development of an acne vulgaris lesion, the earliest morphological change in the pilosebaceous unit is abnormal follicular keratinization. Follicular hyperkeratosis and increased sebum production result in the development of microcomedones, changes in follicular milieu, and intensive growth of P. acnes. P. acnes secretes several proinflammatory products including lipases, proteases, hyaluronidases, and chemotactic factors [2]. The chemotactic factors produced by P. acnes attract cells of the immune system such as neutrophils, monocytes, and lymphocytes [19, 21, 27, 30]. Microcomedones or comedones may then later develop into inflammatory lesions as a result of CD4 + T-cell activation and migration, cytokine production by keratinocytes, macrophages, and neutrophils recruited to the site, hormonal factors and enhanced sebum production [19, 27].
Proinflammatory cytokines (IL-1 alpha, IL-8, and TNF-alpha) are the main responsible mediators of inflammatory acne [30, 46]. It has been shown that P. acnes stimulates cytokine production from lymphocytes, monocytes, and keratinocytes. Both intact P. acnes and isolated cellular factors induce production of proinflammatory cytokines, including TNF-alpha, IL-1alpha, granulocyte/macrophage colony stimulating factor (GM-CSF) [21], IL-1beta, and IL-8 [24, 41].
A proinflammatory cytokine, TNF-alpha is a powerful inducer of the inflammatory response and a key regulator of innate immunity. It enhances major histocompatibility complex (MHC) class I molecule expression on activated T-cells, promotes IL-2-dependent T-cell proliferation, and is a cofactor in B-cell proliferation and immunoglobulin production. Inflammatory responses to TNF-alpha are mediated both directly and through stimulation of the expression of IL-1 and other proinflammatory cytokines [8]. In view of TNF-alpha plays a role in the acne pathogenesis, factors affecting its production may possibly influence the degree of inflammatory response and hence may account for the clinical severity of acne. The cytokine mRNA and protein levels depend on both genetic and environmental factors. Analysis of cytokine gene polymorphisms affecting the production of inflammatory mediators would lead the way to detect a genetic abnormality of cytokine regulation that may play a role in the pathophysiology of the disease. SNPs of certain genes of cytokines and/or cytokine receptors are associated with some human diseases suggesting their likely involvement in the pathogenesis [20]. Even if they do not have striking effects on the development of a disease, they might change penetrance of other important genes, modify disease manifestations and affect their severity.
The TNF-alpha gene is located on chromosome 6 (6p21.3) between HLA-B and DR within the class III region of the major histocompatibility complex [13]. This locus includes two closely linked genes that encode the cytokines TNF-alpha and lymphotoxin alpha (also known as TNF-beta). There are several polymorphisms in the promoter region of the TNF-alpha gene (-850, -863, -857, -575, -375, -308, -274, -238, -237, -162) [4, 45]. The most common polymorphisms are two G to A transition in the promoter at position -238 and -308. These polymorphisms may affect cytokine production [15, 43, 44]. The majority of studies investigating the functional significance of alpha-α promoter polymorphisms have focused on the biallelic SNP TNFA-308 G/A [14, 15, 40, 44]. TNFA-308 A allele has shown to be a stronger transcriptional activator than the common TNFA-308 G allele, in vitro [31, 44]. There are also some studies which show an increased production of TNF-alpha associated with TNFA-308 A allele [1, 11, 33]. Patients with TNFA-308 GA heterozygosity have increased TNF-alpha production [31, 43, 44]. However, in view of the chromosomal localization of the TNF gene, TNF-alpha expression may depend on polymorphisms in the TNF-alpha promoter region or a linkage association with the HLA genotype [15, 36, 43].
TNFA-308 G/A polymorphism have been examined in several autoimmune and inflammatory diseases [3, 6, 7, 12, 20, 28, 38, 40]. However, the results have varied, mainly due to differences in the origin of the studied populations, linkage disequilibrium with other MHC genes or insufficient sample size. This is the first report concerning TNFA-308 G/A polymorphism in acne patients. In the present study, we found that the TNFA-308 GA genotype frequency was statistically significantly increased in patients compared with the controls. However, we did not observe any statistical association between the genotype distributions and severity of acne. In view of acne is a multifactorial disease, it is possible that the severity of acne may be more readily influenced by other factors such as environmental and other genetic elements.
The relationship of acne and various genes has been previously investigated [5, 22, 29, 34, 35, 39]. It has been suggested that human cytochrome P450 1A1 gene (CYP1A1) [35], the steroid 21-hydroxylase gene (CYP21) [34], the epithelial mucin gene (MUC1) [5], and human cytochrome P450c17α gene (CYP17) [22] may be involved in the pathogenesis of acne. Androgen receptor polymorphism (CAG repeat lengths) [39] or Toll-like receptor (TLR)-2, and TLR4 polymorphisms [29] were also investigated in patients with acne, but no association has been shown.
The genotype frequencies of TNFA differ between ethnic groups [6, 20, 25, 28, 32, 37]. This is the first report investigating TNFA-308 G/A polymorphism in acne patients and there is no other study performed for detection of TNFA genotype frequencies in Turkish population with the larger sample size. For this reason, only data of the control subjects were compared with those of some previous studies, to evaluate the effect of ethnicity on our results. The distribution of genotypes of our control subjects was similar with those of some other populations from our country [6, 7, 26], Iran [25], and Korea [28], while it differed from European populations from Italy [32] and England [37], which had higher frequencies of GA genotypes (Table 4).
In summary, we have demonstrated an association between TNFA-308 G/A polymorphism and acne susceptibility. Even in the absence of a confirmed direct functional effect of the polymorphism, our results indicate that TNFA-308 G/A polymorphism may have a role in acne susceptibility in Turkish patients. Further studies with extended samples (from same and different populations) are necessary to confirm our results. In addition, further investigations of other polymorphisms of the TNF gene locus and its association with TNF production in acne patients may be helpful to clarify the pathogenesis of the disease.
References
Abraham LJ, Kroeger KM (1999) Impact of the -308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J Leukoc Biol 66:562–566
Allaker RP, Greenman J, Osborne RH (1987) The production of inflammatory compounds by Propionibacterium acnes and other skin organisms. Br J Dermatol 117:175–183
Allen MH, Wakelin SH, Holloway D, Lisby S, Baadsgaard O, Barker JN, McFadden JP (2000) Association of TNFA gene polymorphism at position-308 with susceptibility to irritant contact dermatitis. Immunogenetics 51:201–205
Allen RD (1999) Polymorphism of the human TNF-alpha promoter—random variation or functional diversity? Mol Immunol 36:1017–1027
Ando I, Kukita A, Soma G, Hino H (1998) A large number of tandem repeats in the polymorphic epithelial mucin gene is associated with severe acne. J Dermatol 25:150–152
Ateş A, Kinikli G, Düzgün N, Duman M (2006) Lack of association of tumor necrosis factor-alpha gene polymorphisms with disease susceptibility and severity in Behçet’s disease. Rheumatol Int 26:348–353
Ates O, Musellim B, Ongen G, Topal-Sarıkaya A (2007) Interleukin-10 and tumor necrosis factor-alpha gene polymorphisms in tuberculosis. J Clin Immunol 11 (Epub ahead of print)
Balkwill FR (1989) Tumour necrosis factor. Br Med Bull 45:389–400
Bataille V, Sneider H, MacGregor AJ, Sasieni P, Spector TD (2002) The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women. J Invest Dermatol 119:1317–1322
Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M (2001) Association between G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry 6:79–82
Bouma G, Crusius JB, Oudkerk Pool M, Kolkman JJ, von Blomberg BM, Kostense PJ, Giphart MJ, Schreuder GM, Meuwissen SG, Pena AS (1996) Secretion of tumour necrosis factor alpha and lymphotoxin alpha in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand J Immunol 43:456–463
Cabrera M, Shaw MA, Sharples C, Williams H, Castes M, Convit J (1995) Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. Exp Med 182:1259–1264
Carroll MC, Katzman P, Alicot EM, Koller BH, Geraghty DE, Orr HT, Strominger JL, Spies T (1987) Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci 84:8535–8539
Cuenca J, Perez CA, Aguirre AJ, Schiattino I, Aguillon JC (2001) Genetic polymorphism at position-308 in the promoter region of the tumor necrosis factor (TNF): implications of its allelic distribution on susceptibility or resistance to diseases in the Chilean population. Biol Res 34:237–241
D’Alfonso S, Richiardi PM (1994) A polymorphic variation in a putative regulation box of the TNF-α promoter region. Immunogenetics 39:150–154
Doshi A, Zaheer A, Stiller MJ (1997) A comparison of current acne grading systems and proposal of a novel system. Int J Dermato l36:416–418
Evans DM, Kırk KM, Nyholt DR, Novac C, Martin NG (2005) Teenage acne is infuenced by genetic factors. Br J Dermatol 52:565–595
Friedman GD (1984) Twin studies of disease heritability based on medical records: application to acne vulgaris. Acta Genet Med Gemellol 33:487–495
Gollnick H (2003) Current concepts of the pathogenesis of acne: implications for drug treatment. Drugs 63:1579–1596
Gottenberg JE, Busson M, Loiseau P, Dourche M, Cohen-Solal J, Lepage V et al (2004) Association of transforming growth factor β1 and tumor necrosis factor alpha polymorphisms with anti-SSB/La antibody secretion in patients with primary Sjogren’s syndrome. Arthritis Rheum 50:570–580
Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E (2004) Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol 150:421–428
He L, Yang Z, Yu H, Cheng B, Tang W, Dong Y, Xiao C (2006) The relationship between CYP17-34T/C polymorphism and acne in Chinese subjects revealed by sequencing. Dermatology 212:338–342
Herane MI, Ando I (2003) Acne in infancy and acne genetics. Dermatology 206:24–28
Jain A, Basal E (2003) Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine 10:34–38
Kamali-Sarvestani E, Nikseresht A, Aflaki E, Sarvari J, Gharesi-Fard B (2007) TNF-alpha, TNF-beta and IL-4 gene polymorphisms in Iranian patients with multiple sclerosis. Acta Neurol Scand 115:161–166
Karahan ZC, Deda G, Sipahi T, Elhan AH, Akar N (2005) TNF-alpha-308G/A and IL-6-174 G/C polymorphisms in the Turkish pediatric stroke patients. Thromb Res 115:393–398
Kim J (2005) Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 211:193–198
Kim TG, Pyo CW, Hur SS, Kim YK, Hwang HY, Youn JI, Kim TY (2003) Polymorphisms of tumor necrosis factor (TNF) alpha and beta genes in Korean patients with psoriasis. Arch Dermatol Res 295:8–13
Koreck A, Kis K, Szegedi K, Paunescu V, Cioaca R, Olariu R, Negru S, Bata-Csorgo Z, Kemeny L, Dobozy A, Szell M (2006) TLR2 and TLR4 polymorphisms are not associated with acne vulgaris. Dermatology 213:267–269
Koreck A, Pivarcsi A, Dobozy A, Kemény L (2003) The role of innate immunity in the pathogenesis of acne. Dermatology 206:96–105
Kroeger KM, Carville KS, Abraham LJ (1997) The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol 34:391–399
Lio D, Annoni G, Licastro F, Crivello A, Forte GI, Scola L, Colonna-Romano G, Candore G, Arosio B, Galimberti L, Vergani C, Caruso C et al (2006) Tumor necrosis factor-alpha-308A/G polymorphism is associated with age at onset of Alzheimer’s disease. Mech Ageing Dev 127:567–571
Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, Mahieu P, Malaise M, De Groote D, Louis R, Belaiche J (1998) Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol 113:401–406
Ostlere LS, Rumsby G, Holownia P, Jacobs HS, Rustin MH, Honour JW (1998) Carrier status for steroid 21-hydroxylase deficiency is only one factor in the variable phenotype of acne. Clin Endocrinol (Oxf) 48:209–215
Paraskevaidis A, Drakoulis N, Roots I, Orfanos CE, Zouboulis CC (1998) Polymorphisms in the human cytochrome P-450 1A1 gene (CYP1A1) as a factor for developing acne. Dermatology 196:171–175
Pociot F, Briant L, Jongeneel CV, Molvig J, Worsaae H, Abbal M et al (1993) Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF- α and TNF-β by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol 23:224–231
Reynard MP, Turner D, Navarrete CV (2000) Allele frequencies of polymorphisms of the tumour necrosis factor-alpha, interleukin-10, interferon-gamma and interleukin-2 genes in a North European Caucasoid group from the UK. Eur J Immunogenet 27:241–249
Roy S, McGuire W, Mascie-Taylor CG, Saha B, Hazra SK, Hill AV, Kwiatkowski D (1997) Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous leprosy. J Infect Dis 176:530–532
Sawaya ME, Shalita AR (1998) Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg 3:9–15
Sullivan KE, Wooten C, Schmeckpeper BJ, Goldman D, Petri MA (1997) A promoter polymorphism of tumor necrosis factor alpha associated with systemic lupus erythematosus in African–Americans. Arthritis Rheum 40:2207–2211
Vowels BR, Yang S, Leyden JJ (1995) Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun 63:3158–3165
Walton S, Wyatt EH, Cunliffe WJ (1988) Genetic control of sebum excretion and acne—a twin study. Br J Dermatol 118:393–396
Wilson AG, de Vries N, Pociot F, di Giovine FS, van der Putte LB, Duff GW (1993) An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and D2 alleles. J Exp Med 177:557–560
Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW (1997) Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA 94:3195–3199
Zhu X, Wang Y, Sun L, Song Y, Sun F, Tang L, Huo Z, Li J, Yang Z (2007) A novel gene variation of TNFalpha associated with ankylosing spondylitis: a reconfirmed study. Ann Rheum Dis 66:1419–1422
Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, Rosenfield R (2005) What is the pathogenesis of acne? Exp Dermatol 14:143–152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baz, K., Emin Erdal, M., Yazıcı, A.C. et al. Association between tumor necrosis factor-alpha gene promoter polymorphism at position -308 and acne in Turkish patients. Arch Dermatol Res 300, 371–376 (2008). https://doi.org/10.1007/s00403-008-0871-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-008-0871-0