Abstract
Ultraviolet (UV)-induced skin cancers, including melanomas and basal/squamous cell carcinomas, occur more frequently in individuals with fair skin than in those with dark skin. Melanin plays an important role in protecting the skin against UV radiation and levels of melanin correlate inversely with amounts of DNA damage induced by UV in human skin of different racial/ethnic groups. The objectives of this study are to review recent progress in our understanding of mechanisms underlying differences in cancer incidence in skins of different colors, particularly between Black and White skin. More specifically, we review DNA damage and apoptosis in various types of skin before and after exposure to UV in our human study protocols using a single UV dose, either one minimal erythema dose (MED) or a similar low dose of 180–200 J/m2. Our data and other published reports indicate that several major mechanisms underlie the increased rates of photocarcinogenesis in fair/light skin. First, UV-induced DNA damage in the lower epidermis (including keratinocyte stem cells and melanocytes) is more effectively prevented in darker skin. Second, rates of repair of DNA damage can differ significantly in individuals. Third, UV-induced apoptosis to remove potentially precancerous cells is significantly greater in darker skin. These results suggest that pigmented epidermis is an efficient UV filter and that UV damaged cells are removed more efficiently in darker skin. The combination of decreased DNA damage and more efficient removal of UV-damaged cells may play a critical role in the decreased photocarcinogenesis seen in individuals with darker skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Responses against environmental stresses including ultraviolet (UV) radiation are diverse in human skin phenotypes among racial/ethnic groups. It is well documented that Black skin (alternatively called “African–American” or “dark” skin) is dramatically more resistant to the damaging effects of UV, including photocarcinogenesis and photoaging, than is White skin (alternatively called “Caucasian” or “light/fair” skin) [1–3]. Indeed, rates of basal/squamous cell carcinomas and melanomas in the United States are 50- and 13-times higher in White skin than in Black skin, respectively [4, 5]. Additionally, the incidence of melanoma worldwide is increasing steadily. Whether the photoprotective effects of melanin are due solely to its role as a UV filter, or whether other properties of melanin are also involved, is an important question to be investigated since there are still major and significant gaps in our understanding of the biosynthesis, structure(s), role(s) and distribution of melanin, and the regulation of those processes, in the skin.
Our research has aimed at providing a scientific basis for public health policies to minimize the risk of photocarcinogenesis and photoaging, interests shared by many other groups. Our studies in this area compared the effects of a single dose of UV on various types of human skin. This single dose was either one minimal erythemal dose (MED) or a standard comparable UV dose of 180–200 J/m2. The subjects studied represented six racial/ethnic groups in six distinct phototypes as defined by Fitzpatrick [3]. In this review, we mainly focus on differences between White skin and Black skin, with the goal of summarizing our current understanding of the effects of UV on human skin, and with emphasis on a novel mechanism identified that removes UV-damaged cells, i.e., melanin-mediated apoptosis.
Melanin distribution and its response to UV
Melanins are synthesized in different types (eumelanin and pheomelanin) and amounts by melanocytes. Szabo [6] and colleagues made the first important observations of the melanocyte distribution in human skin about 50 years ago when they developed an immunohistochemical analysis using DOPA as a melanogenic precursor. They initially examined White skin but later expanded their studies to other racial/ethnic skin types [7]. The sum of those studies [8] provided evidence that the densities and distribution of melanocytes in different types of human skin are quite similar in comparable areas of the body. Szabo and colleagues concluded that the production of different amounts of melanins by melanocytes and their distribution by neighboring keratinocytes resulted in the large differences in pigmentation of the skin among racial/ethnic groups. Indeed, melanin is actively transferred to keratinocytes for distribution towards the surface of the epidermis or in hair shafts. White skin has less melanin and what is produced is typically found in small clusters of melanosomes in keratinocytes while Black skin has more melanin and the melanosomes are distributed individually in keratinocytes (thus absorbing light and UV more efficiently) [9]. Szabo’s[6] group also observed that the density of melanocytes varied according to body location, being the highest on the upper dorsal skin and lower in other areas.
Recent studies by our group [10, 11] and one other [12] reported that the density of melanocytes in various types of racial/ethnic skin is virtually identical, as measured by the expression of melanosomal proteins including tyrosinase, TYRP1, dopachrome tautomerase, gp100, MART-1 and microphthalmia-associated transcription factor (MITF). We also found that the amount of melanin detected by Fontana–Masson stain or by chemical analysis varied greatly and correlated well with visible pigmentation [3]. In other studies, we found that melanin distribution is significantly decreased in skin of the palms and soles and that melanocyte density in those areas is about 10–20% that in skin on other areas of the body [13]. Concerning the melanosome transfer from melanocytes to keratinocytes, both types of cells actively regulate that process [14, 15]. It seems clear at this time that protease activated receptor-2 (PAR-2), which is expressed on keratinocytes [16, 17], as well as keratinocyte growth factor [18], expressed by keratinocytes, play important roles in regulating the transfer of pigment, but much work remains to be done to more fully elucidate that process physiologically.
UV-induced tanning of human skin can be divided into three phases: immediate pigment darkening, persistent pigment darkening and delayed tanning. Several potential mechanisms are probably involved in the phenomenon of UV-induced tanning: the redistribution of existing melanin from the basal layer to the suprabasal layer, changes in the shape and the intracellular localization of melanin and de novo melanin synthesis, among others. We investigated melanin content in the skin and its distribution following UV exposure using fluorescent FS Lamps (National Biological, Twinsburg, OH, USA) and Kodacel filters (Eastman Chemical Products, Kingsport, TN, USA) [10]. The calculated erythemal effective energy (EEE) of the UVB content was 40% and that of the UVA was 60% [19]. We found that changes in the distribution of melanin from the lower layer upwards to the middle layer of the skin were more pronounced in Black skin 1 week after UV exposure than in White skin [10]. Rees’s group developed a novel method to eliminate the effects of erythema on the measurement of melanin pigment and reported that skin tanning peaked at 1 week following UV exposure [20]. They also reported that skin containing lower levels of constitutive pigment had higher degrees of epidermal hyperplasia measured by skin thickening and that this played more of a protective role in UV responses than did increased pigmentation in lighter skin types [21]. Taken together, these studies showed that White skin contains less melanin pigment in upper layers of the epidermis and becomes thicker in response to UV compared to Black skin.
DNA damage in upper and lower epidermis
Kaidbey et al. compared Black and White skin for responses to UV and found that up to five times as much UV reaches the upper dermis of White skin compared with Black skin [1]. They concluded that this is due to increased melanin content, its more efficient distribution and the thickness of the stratum corneum in Black skin. The relationships between skin color, melanin content, race/ethnicity and UV-induced DNA damage have been recently reviewed [22, 23] and the evidence suggests that melanin is significantly involved in photoprotection, but not merely as a sunscreen. Two major types of melanin are produced in human skin (reviewed in [24]), termed eumelanin and pheomelanin. Pheomelanin seems to be toxic in response to UV compared to eumelanin and pheomelanin is found in relatively higher proportions in red-haired sun-sensitive individuals [25]. However, both types of melanin can be a two-edged sword, having beneficial and detrimental effects on melanocytes and on skin tissues exposed to UV [26]. It has been shown that the levels of both types of melanin increase gradually in tandem rather than independently following UV exposure [3, 21, 27]. The most important role of melanin is protection against UV-induced DNA damage, but it also play an important role as an inducer of apoptosis, which will be discussed later.
UV irradiation of human skin results in two major types of DNA lesions; (6-4) photoproducts (6,4PP) and cyclobutane pyrimidine dimers (CPD) [28]. As mentioned above, in one study we compared DNA lesions in the lower epidermis compared with the upper epidermis in response to UV among racial/ethnic groups [19]. That study found that UV-induced DNA damage in the lower epidermis (including keratinocyte stem cells and melanocytes) is more effectively prevented in Black skin compared with White skin even though the one MED of UV used was ∼3.5-fold higher in Black skin. We also found that the melanin content correlated inversely with CPD damage much more significantly in the lower epidermis than that seen in the upper epidermis. These results demonstrate that skin containing more melanin incurs less DNA damage in the lower epidermis and that levels of initial DNA damage in the upper epidermis are similar in skins of different color. Whether facultative pigmentation induced by tanning provides additional photoprotection in White skin is a potentially independent issue to be elucidated in future studies [29–31].
Melanin-related apoptosis
UV-damaged cells often undergo apoptosis [32], presumably to prevent those cells (i.e., cells with potentially risky mutations) from proliferating. We measured apoptotic cells in the skin after exposure to one MED or to the same dose of 180–200 J/m2 UV using TdT-mediated dUTP nick end labeling (TUNEL) assay and immunostaining for proteins involved in apoptosis pathways [19,33]. Our initial expectation was that White epidermis would contain more UV-induced apoptotic cells than Black epidermis since UV-induced DNA damage is significantly higher in White skin as noted above. However, sevenfold more TUNEL-positive cells were observed in Black skin than in White skin after one MED UV exposure [19]. We then speculated that the ∼3.5-fold higher physical UV dose at one MED used for Black skin might have elicited the increase of apoptotic cells and we performed a similar study but using a constant low dose of UV (180–200 J/m2) [19]. Again, whereas almost no apoptotic cells were observed in White skin (Fig. 1), Black skin (Fig. 2) expressed 5.4 ± 2.2 (average number of apoptotic cells ± standard deviation) and 7.9 ± 3.5 TUNEL-positive cells per field at 1 day and at 7 days, respectively, after a constant low UV exposure (Fig. 3a). Increased levels of melanin measured by Fontana–Masson staining correlated with the number of TUNEL-positive cells in the epidermis at 1 day (r 2 = 0.350, p < 0.05) and at 7 days (r 2 = 0.312, p < 0.05) after a similar low dose of UV exposure (Fig. 3b).
A reasonable explanation for those observations is that the melanin within keratinocytes (as determined by the location of apoptotic cells in suprabasal levels of the epidermis) is actively involved in the induction of apoptosis in response to UV. We hypothesized that since the only apparent difference between Black and White skin is the amount and distribution of melanin pigment, as noted above. We then obtained additional evidence to support that hypothesis. Originally, we took biopsies from dorsal skin of the subjects meticulously not to include even vellus hair [3, 23] since follicular epidermis is different from interfollicular epidermis in terms of the amount and distribution of melanin: even White skin contains much melanin around hair follicles. However, we occasionally observed sections containing hair follicles and found that numerous TUNEL-positive cells were present in outer root sheath cells, which were located around hair shafts and contained melanin (Fig. 4). We also performed ex vivo studies using reconstructed three-dimensional human skin equivalents (termed MelanoDerm®) which contained melanocytes derived from Black or White donors, but used keratinocytes from the same Hispanic donor [19]. The differences in amount and distribution of melanin are significant among these skin composites, and accurately represent typical skin morphologies of those types of skin [34]. More apoptotic cells were found in Black skin equivalents than in White skin equivalents 2 days after low doses of UVB radiation [19], which demonstrates that the melanin is responsible since the keratinocyte populations were identical.
The results from other studies also support the concept of melanin-mediated apoptosis as seen in in vivo human [35], in vivo animal [36] and in vitro studies [37, 38]. Although one White and one Black subject showed similar numbers of sunburn cells, characterized as cells with condensed nuclei and with no cytoplasmic substances by hematoxylin–eosin staining, after irradiation with four MED UV, melanin pigment was more apparent in the sunburn cells than in the surrounding cells [35]. A study in mice showed that eumelanin or pheomelanin elicited apoptosis in skin exposed to UV [36]. Finally, in vitro studies show that melanophages, i.e., macrophages containing melanin particles, undergo cell death after UV exposure [37] and that melanocytes in White skin are likely to prevent sunburn cell formation [38]. Taken together, it is clear that the presence of melanin in cells facilitates the apoptotic effect of UV. It might be possible that low doses of UV cause melanin-specific photothermolysis, which could be considered another form of photoprotection, as 351 nm pulse lasers cause highly selective injury to melanocytes containing melanosomes [39].
Pathways involved in melanin-related apoptosis
We are now focusing on investigating various factors that are involved in melanin-induced apoptosis to test whether that results from the generation of heat from the absorbed UV energy or whether other properties of melanin are actively involved.
The oncogene p53 plays important roles in responses not only to DNA damage and its repair process but also to UV-induced apoptosis through its phosphorylation at Ser-46 [40]. As expected, more p53 protein accumulated in the nuclei of White skin than of Black skin since p53 nuclear accumulation is primarily associated with the process of overall DNA damage and its repair [19]. On the contrary, p53 phosphorylated at Ser-46 was not seen in White skin after UV exposure, but similar numbers of TUNEL-positive cells were readily seen in Black skin, suggesting that Ser-46 phosphorylated p53 is actively involved in UV-induced apoptosis [19]. The apurinic–apyrimidinic endonuclease/redox effector factor-1 (APE/Ref-1) is a DNA-repair endonuclease and is associated with the induction of apoptosis through p53: over-expression of APE/Ref-1 increases the ability of p53 to induce apoptosis and down-regulation of APE/Ref-1 reduces that ability [41]. We have shown that the nuclear translocation of APE/Ref-1 is more readily observed in Black skin than in White skin after UV exposure, suggesting that the APE/Ref-1-p53 pathway is involved in UV-induced apoptosis [33].
In contrast, we have shown that staining for active/cleaved caspase-3 was highest 1 day after UV exposure and that cleaved caspase-3 returned to baseline levels by day 7 with no significant difference between White and Black skin. Those results suggest that melanin-induced apoptosis occurs through caspase-3 independent pathways [19].
One of the most important receptors on melanocytes is the melanocortin 1 receptor (MC1R), which is a member of the G-protein-coupled receptor family. It is well documented that activation of MC1R, either by α-MSH or ACTH, increases the intracellular cAMP concentration. That in turn stimulates the activity of various transcription factors, including MITF, and melanogenic enzymes, including tyrosinase, which modulate the biosynthesis of melanins [42]. Mutations in MC1R elicit the red hair light skin phenotype, which correlates with increased risk for skin cancer. We have recently reported that Black skin has higher absolute levels of MC1R350 [43], a new human MC1R isoform distinct from the MC1R315 form reported by Tan et al. [44]. The function of the novel MC1R350 isoform needs to be further elucidated, but it suppresses the expression of MITF and tyrosinase [43], which suggests that Black skin has a greater amount of overall MC1R and that MC1R may be involved with melanin-induced apoptosis in response to UV.
Conclusions
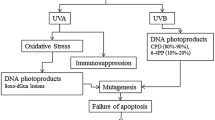
In summary, we conclude that several distinct mechanisms act in concert to result in the dramatic differences in rates of photocarcinogenesis in Black skin and White skin (Fig. 5). First, UV-induced DNA damage in the lower epidermis (which includes melanocytes and keratinocyte stem cells) is not effectively prevented in White skin, although DNA damage in the upper epidermis is similar between the two ethnic groups, suggesting that pigmented epidermis is an efficient UV filter. Second, less efficient DNA repair caused by the severe damage from UV in lighter skin may result in inherited mutations in critical genes involved in photocarcinogenesis. Third, UV-induced apoptosis is less frequent in White skin after low doses of UV, although it is significant in Black skin, suggesting that Black skin removes UV-damaged cells by apoptosis more effectively. The combination of decreased DNA damage and more efficient removal of damaged cells plays an important role in the decreased photocarcinogenesis seen in Black skin. There may of course be additional important factors that play roles, e.g., the more immunosuppressed condition of White skin in response to UV may trigger carcinogenesis (as shown in mouse models [45], although further studies will be necessary to examine those mechanisms.
Abbreviations
- 6,4PP:
-
(6-4) Photoproducts
- CPD:
-
Cyclobutane pyrimidine dimers
- MC1R:
-
Melanocortin 1 receptor
- MED:
-
Minimal erythema dose
- MITF:
-
Microphthalmia transcription factor
- PAR-2:
-
Protease activated receptor-2
- PI:
-
Propidium iodide
- TUNEL:
-
TdT-mediated dUTP nick end labeling
- UV:
-
Ultraviolet
References
Kaidbey KH, Agin PP, Sayre RM, Kligman AM (1979) Photoprotection by melanin—a comparison of black and Caucasian skin. J Am Acad Dermatol 1:249–260
Kollias N, Sayre RM, Zeise L, Chedekel MR (1991) Photoprotection by melanin. J Photochem Photobiol B 9:135–160
Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ (2003) UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. Faseb J 17:1177–1179
Halder RM, Bridgeman-Shah S (1995) Skin cancer in African Americans. Cancer 75:667–673
English DR, Armstrong BK, Kricker A, Fleming C (1997) Sunlight and cancer. Cancer Causes Control 8:271–283
Szabo G (1954) The number of melanocytes in human epidermis. Br Med J 1:1016–1017
Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB (1969) Racial differences in the fate of melanosomes in human epidermis. Nature 222:1081–1082
Staricco RJ, Pinkus H (1957) Quantitative and qualitative data on the pigment cells of adult human epidermis. J Invest Dermatol 28:33–45
Konrad K, Wolff K (1973) Hyperpigmentation, melanosome size, and distribution patterns of melanosomes. Arch Dermatol 107:853–860
Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ (2005) Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol 124:1326–1332
Yamaguchi Y, Hearing VJ (2006)Melanocyte distribution and function in human skin: effects of UV radiation, chap 6. In: Hearing VJ, Leong SPL (eds) From melanocytes to malignant melanoma: the progression to malignancy. Humana, Totowa pp 101–115
Alaluf S, Barrett K, Blount M, Carter N (2003) Ethnic variation in tyrosinase and TYRP1 expression in photoexposed and photoprotected human skin. Pigment Cell Res 16:35–42
Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, Hearing VJ (2004) Mesenchymal–epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol 165:275–285
Minwalla L, Zhao Y, Le Poole IC, Wickett RR, Boissy RE (2001) Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J Invest Dermatol 117:341–347
Virador VM, Muller J, Wu X, Abdel-Malek ZA, Yu ZX, Ferrans VJ, Kobayashi N, Wakamatsu K, Ito S, Hammer JA, Hearing VJ (2002) Influence of alpha-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. Faseb J 16:105–107
Scott G, Leopardi S, Parker L, Babiarz L, Seiberg M, Han R (2003) The proteinase-activated receptor-2 mediates phagocytosis in a Rho-dependent manner in human keratinocytes. J Invest Dermatol 121:529–541
Babiarz-Magee L, Chen N, Seiberg M, Lin CB (2004) The expression and activation of protease-activated receptor-2 correlate with skin color. Pigment Cell Res 17:241–251
Cardinali G, Ceccarelli S, Kovacs D, Aspite N, Lotti LV, Torrisi MR, Picardo M (2005) Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J Invest Dermatol 125:1190–1199
Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ (2006) Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. Faseb J 20:1486–1488
Oh C, Hennessy A, Ha T, Bisset Y, Diffey B, Rees JL (2004) The time course of photoadaptation and pigmentation studied using a novel method to distinguish pigmentation from erythema. J Invest Dermatol 123:965–972
Hennessy A, Oh C, Rees J, Diffey B (2005) The photoadaptive response to ultraviolet exposure in human skin using ultraviolet spectrophotometry. Photodermatol Photoimmunol Photomed 21:229–233
Giacomoni PU (1995) Open questions in photobiology III. Melanin and photoprotection. J Photochem Photobiol B 29:87–89
Beer JZ, Hearing VJ (2007) Skin color, melanin, race/ethnicity and UV-induced DNA damage. Eur Soc Photobiol (in press)
Wakamatsu K, Ito S (2002) Advanced chemical methods in melanin determination. Pigment Cell Res 15:174–183
Cesarini JP (1988) Photo-induced events in the human melanocytic system: photoaggression and photoprotection. Pigment Cell Res 1:223–233
Hill HZ, Li W, Xin P, Mitchell DL (1997) Melanin: a two edged sword? Pigment Cell Res 10:158–161
Wakamatsu K, Ito S (2006) Evaluation of melanin-related metabolites as markers of solar ultraviolet-B radiation. Pigment Cell Res 19:460–464
Young AR, Chadwick CA, Harrison GI, Hawk JL, Nikaido O, Potten CS (1996) The in situ repair kinetics of epidermal thymine dimers and 6-4 photoproducts in human skin types I and II. J Invest Dermatol 106:1307–1313
Hemminki K, Xu G, Kause L, Koulu LM, Zhao C, Jansen CT (2002) Demonstration of UV-dimers in human skin DNA in situ 3 weeks after exposure. Carcinogenesis 23:605–609
Sheehan JM, Cragg N, Chadwick CA, Potten CS, Young AR (2002) Repeated ultraviolet exposure affords the same protection against DNA photodamage and erythema in human skin types II and IV but is associated with faster DNA repair in skin type IV. J Invest Dermatol 118:825–829
Young AR, Potten CS, Sheehan-JM (2002) Epidermal DNA repair under repeated exposure conditions is complex. J Invest Dermatol 119:700–702
Wikonkal NM, Brash DE (1999) Ultraviolet radiation induced signature mutations in photocarcinogenesis. J Investig Dermatol Symp Proc 4:6–10
Takahashi K, Hoashi T, Yamaguchi Y, Mutsuga N, Suzuki I, Vieira WD, Hearing VJ (2006) UV increases the nuclear localization of apurinic/apyrimidinic endonuclease/redox effector factor-1 in human skin. J Invest Dermatol 126:2723–2726
Yoon TJ, Lei TC, Yamaguchi Y, Batzer J, Wolber R, Hearing VJ (2003) Reconstituted 3-dimensional human skin of various ethnic origins as an in vitro model for studies of pigmentation. Anal Biochem 318:260–269
Olson RL, Gaylor J, Everett MA (1974) Ultraviolet-induced individual cell keratinization. J Cutan Pathol 1:120–125
Takeuchi S, Zhang W, Wakamatsu K, Ito S, Hearing VJ, Kraemer KH, Brash DE (2004) Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc Natl Acad Sci USA 101:15076–15081
Johnson BE, Mandell G, Daniels F Jr (1972) Melanin and cellular reactions to ultraviolet radiation. Nat New Biol 235:147–149
Cario-Andre M, Pain C, Gall Y, Ginestar J, Nikaido O, Taieb A (2000) Studies on epidermis reconstructed with and without melanocytes: melanocytes prevent sunburn cell formation but not appearance of DNA damaged cells in fair-skinned caucasians. J Invest Dermatol 115:193–199
Anderson RR, Parrish JA (1983) Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 220:524–527
Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y (2000) p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849–862
Gaiddon C, Moorthy NC, Prives C (1999) Ref-1 regulates the transactivation and pro-apoptotic functions of p53 in vivo. Embo J 18:5609–5621
Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ (2000) The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res 13 Suppl (8):156–162
Rouzaud F, Costin GE, Yamaguchi Y, Valencia JC, Berens WF, Chen KG, Hoashi T, Bohm M, Abdel-Malek ZA, Hearing VJ (2006) Regulation of constitutive and UVR-induced skin pigmentation by melanocortin 1 receptor isoforms. Faseb J 20:1927–1929
Tan CP, McKee KK, Weinberg DH, MacNeil T, Palyha OC, Feighner SD, Hreniuk DL, Van Der Ploeg LH, MacNeil DJ, Howard AD (1999) Molecular analysis of a new splice variant of the human melanocortin-1 receptor. FEBS Lett 451:137–141
Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T (2005) Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med 201:173–179
Acknowledgments
This work was supported in part by the Lydia O’Leary Memorial Foundation, by a SHISEIDO Grant for Science Research, by the Rhoto award, by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (Japan), by the FDA Office of Science and Center for Devices and Radiological Health, and by the Intramural Research Program of the NIH, National Cancer Institute. The authors are grateful to Drs. Taketsugu Tadokoro, Kaoruko Takahashi, Sharon A. Miller, Barbara Z. Zmudzka and Sergio G. Coelho for their helpful discussions of this work.
Conflict of interest statement
None of the authors has any potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, Y., Beer, J.Z. & Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: factors influencing the incidence of skin cancer. Arch Dermatol Res 300 (Suppl 1), 43–50 (2008). https://doi.org/10.1007/s00403-007-0807-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-007-0807-0