Abstract
Introduction
The need for precise quantification of the glenoid defect should be emphasized in the choice of surgery for bony Bankart lesion especially in its critical values of 16% to 25. The study aims to verify the validity of bare spot method for arthroscopic quantification of glenoid bone defect using several varieties of posterior portal location.
Materials and methods
Two intact cadaveric glenoids were prepared for the study. The greatest anteroposterior diameter of the perfect circle concept of the glenoid is identified and center of the circle is marked as glenoid bare spot with metal marker. Sixteen percent and 25% defect were sequentially created using a saw at 0° axis parallel to the longitudinal axis of the glenoid. These were confirmed by 3D CT glenoid scan based on glenoid rim distances. Each glenoids were mounted on Sawbone dome holder model simulating neutral version.
Quantification of Glenoid bone defects were sequentially measured by glenoid bare spot method arthroscopically by 5 shoulder arthroscopy trained surgeons in 5 varieties of posterior portals in 5 cycles. Paired sample t test was done for arthroscopic over CT scan method of glenoid bone loss quantification. One way ANOVA for portal location analysis was done.
Results
Glenoid bare spot method significantly underestimates 16% and 25% glenoid bone defect to 9% ± 2 (P < 0.001) and 18% ± 2 (P < 0.001), respectively, compared to 3D CT scan method. There was good intra-class correlation coefficient of 0.97 for inter-rater reliability. There was no significant difference in quantification in between five portal sites by one-way ANOVA (P > 0.05).
Conclusions
Arthroscopic glenoid bare spot method using the anterior viewing portal significantly underestimates glenoid bone loss in critical margin degrees of decision making in shoulder instability surgery. Minimal variation of posterior portal location for the calibrated probe does not cause significant difference in Glenoid bone loss quantification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Major determinant of shoulder instability surgery is the recurrence rates after the surgery. Increasing number of literatures are revealing high recurrence rates after arthroscopic soft tissue stabilization of anterior shoulder dislocation with bony Bankart lesion [1]. Burkhart et al. examined 194 consecutive patients undergoing soft tissue Bankart repair revealing 67% and 4% recurrence rates for patient with and without significant bone loss, respectively [2]. Bigliani et al. noted 12% of 22 shoulder instability recurrence at a 30 months mean follow-up after soft tissue Bankart lesion repair [3]. Provencher et al. also describes 14% recurrence rates of 21 patients with > 25% glenoid bone defect after arthroscopic stabilization with suture anchors.
There are several ways to quantitate glenoid bone loss in anterior unstable shoulders. A three-dimensional (3D) reconstructed computed tomography (CT) scan in enface view of the glenoid with humerus reduction is considered to be the most reliable imaging modality in glenoid defect quantification [4]. There are several methods of quantification using the best fit circle concept as described by Huysman et al. [5]. An accurate but simple and practical method of quantifying the glenoid bone defect in 3D reconstructed CT scan images of the glenoid is the anterior posterior (AP) distance from bare area method [6, 7].
Arthroscopic method introduced by Burkhart et al. in 2002 was based on the 56 patients and 10 cadavers with consistently present and central location of the glenoid bare spot (GBS) that quickly gained acceptance. They use the concept that the inferior 2/3 of the glenoid forms a perfect circle and the GBS is consistently in the center of the circle. They measure the posterior radius distance from the GBS to the posterior-most rim of the glenoid and the anterior distance of the glenoid bare spot to the anterior bone defect of a bony Bankart lesion [8]. However, several authors insist that the GBS is not universally present and if ever present, is not always located geometrically in the center of the perfect fit circle of the inferior 2/3 of the glenoid and, therefore, suggest that the arthroscopic glenoid bare spot method is inaccurate [5, 9,10,11]. However, it should be emphasized that most study that contrasts that of Burkhart’s findings were done on elderly cadavers and, therefore, may have degenerative and artificial changes to the articular cartilage due to old age and embalming process, respectively [12].
The study aims to rise a construct of validity in measuring the bone defect in bony Bankart lesions using the arthroscopic GBS method. The researchers also wanted to know if certain variability in posterior portal creation for the working portal influence the GBS method for calculation of glenoid bony defect. We hypothesize that arthroscopic bare spot method accurately quantify glenoid bone loss in 16% and 25% true glenoid bone defect. We also hypothesize that variation of the placement of posterior portal for the measuring probe significantly differ the quantification.
Materials and methods
IRB exemption was obtained prior to the study. Two glenoids from male cadaver were dissected off its soft tissue and osteotomized at the neck level of the scapula. The glenoids are sequentially examined and tested. The greatest anteroposterior diameter of the perfect circle concept of the glenoid is grossly identified and the center of the circle is marked as glenoid bare spot (GBS) with metal marker with equal distance from anterior and posterior margins of the circle. Gross measurements were done using digital caliper.
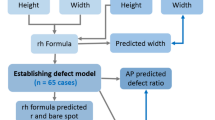
A 16% anterior bone defect was created using a saw at 0° axis in line with longitudinal axis of the glenoid. CT scans were obtained and subsequent 3D reconstructed quantification of glenoid bone loss based on glenoid rim distances was done using MIMICS (Mimics Research 17.0, Materialize, Leuven, Belgium). A perfect circle was approximated on an enface view 3D reconstructed glenoids. The distance posterior (Dp) and anterior (Da) from the metal marked center GBS was measured accordingly and the percentage bone defect was quantified using the following formula: \(\% \;{\text{glenoid~bone~loss~}} = \frac{{{\text{Dp}} - {\text{Da}}}}{{{\text{Dp}} \times 2}} \times 100\% \;{\text{glenoid~bone~loss~}} = \frac{{{\text{Dp}} - {\text{Da}}}}{{{\text{Dp}} \times 2}} \times 100\) (Fig. 1). This method of calculation was applied according to the previous study [13].
The glenoids are then mounted in neutral position on Sawbone dome holder model. Portal sites were then created on the plastic cover of the model. The anterior portal as the viewing portal was created mimicking a true anterior portal in surgical arthroscopy. It is positioned at 2 o’ clock of right glenoid (10 o’ clock of left glenoid) face. Five posterior portal were made for the 2 mm increments marked probe entry points. The first posterior portal is made recreating the posterior portal located 2 cm inferior and 2 cm medial to the posterolateral edge of the acromion of a true arthroscopic shoulder surgery. It is oriented at 10 o’ clock of the right glenoid face (2 o’ clock for left glenoid). Four other varieties of posterior portal were created 10 mm superior, lateral, inferior and medial from the first portal (Fig. 2).
Quantification of glenoid bone defects were sequentially measured by “glenoid bare spot method” arthroscopically by 5 shoulder arthroscopy trained orthopaedic surgeons using the five varieties of posterior portals in five cycles. In this method, it is assumed that the inferior part of the glenoid forms a perfect circle, and that the bare spot was located at its center. A 30° Conmed-Linvatech Arthoscope was inserted on the anterior viewing portal. A calibrated arthroscopic probe was inserted through the posterior portals to measure distances. The anterior distance (Da) is measured by hooking the probe on the anterior defect and reading the distance (Dʹa) corresponding to the level of the marked GBS. The posterior distance (Dp) is measured by placing the tip of the probe on the GBS and reading the distance (Dpʹ) at the level of the posterior-most margin of glenoid. The percentage of bone defect was quantified using the following formula: \({\text{\% glenoid~bone~loss~}} = \frac{{{\text{Dp}^{\prime}} - {\text{D}^{\prime}{\rm a}}}}{{{\text{Dp}^{\prime}} \times 2}} \times 100\) (Fig. 3) according to the previous study [13].
Twenty-five percent defect was then calculated and created to the glenoid and the process of 3D CT scan quantification and arthroscopic “glenoid bare spot method” of quantification are then repeated.
Statistical analysis
SPSS version 23 was used for statistical analysis. A student’s paired sample t test was used to analyze percent bone defect derived from arthroscopic glenoid bare area and 3D CT scan method of glenoid bone loss quantification at every posterior portal. Intra-class correlation coefficient for inter-observer reliability was done. One-way ANOVA was used to analyze whether the variable portals influence the quantification of glenoid bone loss. The threshold for significant findings (P value) was set at P < 0.05.
Results
There was no difference in quantification of 16% and 25% glenoid bone loss with the gross measurement with a caliper and 3D CT scan anteroposterior distance method. Glenoid bare spot method significantly underestimates 16% and 25% glenoid bone defect to 9% ± 2 (P < 0.001) and 18% ± 2 (P < 0.001), respectively, based on the means of all measures for both glenoids on all five varieties of portals measured by all five surgeons. The means for each portals in 16% defect for both glenoids are 9.2 ± 1.75 SD for the first portal, 8 ± 1.76 SD for the second portal, 6.7 ± 1.70 SD for the third portal, 10.2 ± 2.48 SD for the fourth portal, and 10.4 ± 1.64 SD for the fifth portal. The mean percent bone loss arthroscopic quantification by the five surgeons for 16% defect for the left and right glenoids are shown in Table 1. The means for each portals in 25% defect for both glenoids are 17.8 ± 1.48 SD for the first portal, 16.5 ± 1.96 SD for the second portal, 15.9 ± 1.52 for the third portal, 18.0 ± 0.94 SD for the fourth portal, and 19.7 ± 1.7 SD for the fifth portal. The mean percent bone loss arthroscopic quantification for 25% defect for the left and right glenoids are shown in Table 2. Inter-rater reliability measurement revealed excellent average measures of intra-class correlation coefficient of 0.977.
All the five varieties of posterior portal locations from portal 1–5 revealed no significant difference in arthroscopic quantification of glenoid bone defect as shown on one-way ANOVA for 16% (P = 0.159, P = 0.162, P = 0.094, P = 0.816, P = 0.273, respectively) and 25% (P = 0.424, P = 0.883, P = 0.849, P = 1.000, P = 0.094, respectively) glenoid bone defects (Fig. 4).
Portal location effect on the arthroscopic GBS method on quantification glenoid bone defect in gross and CT scan measured 16% (a) and 25% bone defect glenoid (b). There was no significant difference in between right and left glenoid quantification and there is no significant difference of value between every portal for both defect size (P > 0.05)
Discussion
The results of the study opposed our hypothesis. Arthroscopic glenoid bare spot method significantly underestimate the true glenoid bone defect both in the critical value of 16% and 25% defect on both the right and left glenoids. This contrast with the study done by Provencher et al., where they noted that arthroscopic quantification of glenoid bone defect by glenoid bare spot method on cadaveric specimen accurately estimates true 12.5 and 25% glenoid bone defect in 14 cadaveric shoulder at 0° to the long axis of the glenoid [14]. They, however, did not measure using an arthroscope, instead they exposed the whole scapula and glenoid and used a calibrated probe with digital calipers which are usually not applicable in real setting of arthroscopic surgery. In addition, their study used the cadaveric glenoid bare spot as reference point of measurement which contrast our method of making the center of the perfect circle concept as the reference. Their study also revealed no significant difference of their quantification using a 2 or a 3 o’ clock posterior portal. In our study, the first portal placed in a standard posterior portal of a real arthroscopy setting at 10o’ clock of the right glenoid (2 o’ clock on left glenoid) with the other 4 variable posterior portals placed 10 mm superior, lateral, inferior to the first portal does not significantly differ in measurements.
Several studies revealed different critical value in percent of glenoid bone loss severity that necessitates a bony reconstruction procedure. Burkhart describes significant bony Bankart lesion as having a “inverted pear” shaped glenoid on arthroscopic view [2]. Most authors recommends 25% as the critical value for bone grafting [3, 6, 15]. Several studies, however, describes 20% as critical value of bone defect [16,17,18]. In a cadaveric study by Shin et al., more than 15% glenoid bone defect was considered as critical value that which a soft tissue repair deemed insufficient to restore glenohumeral translation, restricts rotational range of motion, and leads to abnormal humeral head position [19]. In our study, we use 16% and 25% defect as the critical margin of bone defect.
Many authors suggested that the arthroscopic bare spot method is unreliable based on inconsistent landmarks. However, Burkhart’s study emphasize that the landmark, glenoid bare spot for measurement of the anteroposterior distance is always present and always located at its center [8]. Most authors emphasize that such landmark is not always present in most of the cadaveric specimens and if present, is not located almost always centrally in the inferior 2/3 of the glenoid [5, 9,10,11]. Our study eliminated this questionable factor of centrality of the glenoid bare spot both in arthroscopic and CT scan measurement by creating and marking the center of the circle as the glenoid bare spot.
The cadaveric study by Provencher et al. revealed overestimation of glenoid bone loss using arthroscopic anterior–posterior distance measurement [14]. They, however, artificially created a glenoid bone defect oriented 45° to the longitudinal axis of the glenoid. However, together with other studies [17, 20, 21], we believe that most bony Bankart lesion are oriented parallel or at 0° orientation to the long axis of the glenoid.
The authors believe that one factor for the inaccurate quantification of the glenoid bone defect size is the perspective distortion between the probe and the accurate plane to measure the distance from anterior rim to the glenoid bare spot and from the glenoid bare spot to the posterior rim. In optics, perspective distortion is a transformation of an object and its surrounding area due to the relative scale of nearby and distant features. This factor is dependent to the focal length of the lens used and to the distance and angles between the objects measured and between the objects and the lens. In an effort to demonstrate this factor, we objectively measure from five of variants of posterior portal location for the arthroscopic calibrated probe. All quantification from all variants significantly underestimates the true glenoid bone defect. In addition, the cone of vision of the lens of the scope should be as much as possible perpendicular to both the points of measurements on the probe and on the glenoid which is not usually possible in an actual anatomical arthroscopic bare spot method due to the presence of the humeral head. In actual shoulder arthroscopy of patients, posterior portal placement and precise arthroscope location does vary in between surgeons and glenoid version do vary in between patients. This factor also question the validity of arthroscopic bare spot method of glenoid bone defect quantification.
In addition, the inferior 2/3 of normal glenoid only measures to 24–28 mm in greatest anteroposterior distance. Calculating percent values from 24 mm denominator would mean that a millimeter difference could mean 4–5% difference in glenoid bone defect quantification. In addition, arthroscopic bare spot method is limited only to 1 mm increment of measurement.
We also noted that although there was some variability of the per cent glenoid bone defect in between the raters, these were not significant. Statistical significance was set to 95 percentiles with significant P value set as < 0.05. Inter-rater reliability of overall quantification revealed an overall intra-class correlation coefficient of 0.977.
There are several points of advantages of this study. (1) The posterior portals are placed in a more consistent manner rather than that using either a soft shoulder model or a cadaver. (2) The scope is positioned in a constant location at the antero-superior portion of the glenoid.
There are limitations of the current study. First, this is a cadaveric study with small number of sample. The specimen was mounted on an arthroscopic shoulder model and thus factors from actual version of glenoid, and effect of presence of other soft tissue such as cartilage and labrum, humerus and arthroscopic milieu were not included. Second, this pilot study include only male gender and Asian ethnic cadaver which did not consider the effect of gender, ethnicity and body size due that may affect the glenoid size [22]. Our study represent the pilot study, hence it does not necessitate traditional power analysis despite the small number of sample [9]. Future study on a clinical setting with larger sample size of observers may further validate the result of the study.
Conclusion
Arthroscopic glenoid bare spot method may underestimates glenoid bone loss by 9 and 18% in a setting of 16% and 25% of critical glenoid defect, respectively. This additional knowledge may help surgeons in managing anterior shoulder instability. The authors recommend not relying on arthroscopic method of quantification or estimation of bone loss using glenoid bare spot method.
References
Boileau P, Villalba M, Hery JY, Balg F, Ahrens P, Neyton L (2006) Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Jt Surg Am 88(8):1755–1763. https://doi.org/10.2106/JBJS.E.00817
Burkhart SS, De Beer JF (2000) Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill–Sachs lesion. Arthroscopy 16(7):677–694
Bigliani LU, Newton PM, Steinmann SP, Connor PM, McLlveen SJ (1998) Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med 26(1):41–45. https://doi.org/10.1177/03635465980260012301
Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS (2013) 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res 471(4):1251–1256. https://doi.org/10.1007/s11999-012-2607-x
Huysmans PE, Haen PS, Kidd M, Dhert WJ, Willems JW (2006) The shape of the inferior part of the glenoid: a cadaveric study. J Shoulder Elbow Surg 15(6):759–763. https://doi.org/10.1016/j.jse.2005.09.001
Provencher MT, Bhatia S, Ghodadra NS, Grumet RC, Bach BR Jr, Dewing CB, LeClere L, Romeo AA (2010) Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Jt Surg Am 92(Suppl 2):133–151. https://doi.org/10.2106/JBJS.J.00906
Sugaya H, Moriishi J, Kanisawa I, Tsuchiya A (2006) Arthroscopic osseous Bankart repair for chronic recurrent traumatic anterior glenohumeral instability. Surgical technique. J Bone Jt Surg Am 88(Suppl 1 Pt 2):159–169. https://doi.org/10.2106/JBJS.F.00319
Burkhart SS, Debeer JF, Tehrany AM, Parten PM (2002) Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy 18(5):488–491. https://doi.org/10.1053/jars.2002.32212
Saintmard B, Lecouvet F, Rubini A, Dubuc JE (2009) Is the bare spot a valid landmark for glenoid evaluation in arthroscopic Bankart surgery? Acta Orthop Belg 75(6):736–742
Barcia AM, Rowles DJ, Bottoni CR, Dekker TJ, Tokish JM (2013) Glenoid bare area: arthroscopic characterization and its implications on measurement of bone loss. Arthroscopy 29(10):1671–1675. https://doi.org/10.1016/j.arthro.2013.06.019
Kralinger F, Aigner F, Longato S, Rieger M, Wambacher M (2006) Is the bare spot a consistent landmark for shoulder arthroscopy? A study of 20 embalmed glenoids with 3-dimensional computed tomographic reconstruction. Arthroscopy 22(4):428–432. https://doi.org/10.1016/j.arthro.2005.12.006
Burkhart SS (2007) The bare spot of the glenoid. Arthroscopy 23(4):449. https://doi.org/10.1016/j.arthro.2006.12.018 (author reply 449–450)
Chuang TY, Adams CR, Burkhart SS (2008) Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy 24(4):376–382. https://doi.org/10.1016/j.arthro.2007.10.008
Provencher MT, Detterline AJ, Ghodadra N, Romeo AA, Bach BR Jr, Cole BJ, Verma N (2008) Measurement of glenoid bone loss: a comparison of measurement error between 45 degrees and 0 degrees bone loss models and with different posterior arthroscopy portal locations. Am J Sports Med 36(6):1132–1138. https://doi.org/10.1177/0363546508316041
Yamamoto N, Muraki T, Sperling JW, Steinmann SP, Cofield RH, Itoi E, An KN (2010) Stabilizing mechanism in bone-grafting of a large glenoid defect. J Bone Jt Surg Am 92(11):2059–2066. https://doi.org/10.2106/JBJS.I.00261
Yamamoto N, Itoi E, Abe H, Kikuchi K, Seki N, Minagawa H, Tuoheti Y (2009) Effect of an anterior glenoid defect on anterior shoulder stability: a cadaveric study. Am J Sports Med 37(5):949–954. https://doi.org/10.1177/0363546508330139
Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A (2003) Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Jt Surg Am 85-A(5):878–884
Itoi E, Lee SB, Berglund LJ, Berge LL, An KN (2000) The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Jt Surg Am 82(1):35–46
Shin SJ, Koh YW, Bui C, Jeong WK, Akeda M, Cho NS, McGarry MH, Lee TQ (2016) What is the critical value of glenoid bone loss at which soft tissue Bankart repair does not restore glenohumeral translation, restricts range of motion, and leads to abnormal humeral head position? Am J Sports Med 44(11):2784–2791. https://doi.org/10.1177/0363546516656367
Itoi E, Lee SB, Amrami KK, Wenger DE, An KN (2003) Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med 31(1):112–118. https://doi.org/10.1177/03635465030310010301
Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y (2005) Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med 33(6):889–893. https://doi.org/10.1177/0363546504271521
Piponov HI, Savin D, Shah N, Esposito D, Schwartz B, Moretti V, Goldberg B (2016) Glenoid version and size: does gender, ethnicity, or body size play a role? Int Orthop 40(11):2347–2353. https://doi.org/10.1007/s00264-016-3201-8
Acknowledgements
We thank Tae-Hyun Ha for providing illustrations. This research was supported by the convergence technology development program for bionic arm through the National Research Foundation of Korea (NRF) funded by the Ministry of Science & ICT (No. 2014M3C1B2048422). IRB: Exemption number S2017-0841-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kho, J., Kholinne, E., Lim, S. et al. Arthroscopic bare spot method underestimates true bone defect in bony Bankart lesion. Arch Orthop Trauma Surg 139, 1269–1275 (2019). https://doi.org/10.1007/s00402-019-03195-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-019-03195-7