Abstract
Objective
The aim of this observational study was to investigate the risk factors of postoperative valgus malalignment after mobile-bearing medial unicompartmental knee arthroplasty (UKA).
Methods
We retrospectively evaluated radiographic and surgical characteristics in 122 consecutive Oxford phase 3 UKAs. According to postoperative hip–knee–ankle angle (HKAA), 24 knees were sorted into group valgus with HKAA > 180° and 98 knees were sorted into group non-valgus with HKAA ≤ 180°. Logistic regression was performed to analyze risk factors including age, gender, BMI, side, preoperative limb alignment HKAA, preoperative LDFA, MPTA, FTFA, thickness of polyethylene bearing insert, tibial prothesis size, femoral prothesis size, medial tibial cut thickness, thickness of distal femoral mill, prothesis angle of coronal, and sagittal plane.
Results
The mean mechanical preoperative HKAA of 174.39°±4.23° was corrected to 178.18°±3.49° postoperatively (t = − 13.45, p = 0.000). The mean of postoperative HKAA in valgus group and non-valgus group was 183.45 ± 2.21° and 176.88 ± 2.35°, respectively (t = 12.44, p = 0.000). After statistical analysis with univariate analysis, eight risk factor variables among 16 independent variables were identified as potential predictors with p value ≤ 0.1. Multivariate logistic regression analysis for these eight potential predictors revealed that tibial cut (p = 0.046), LDFA (p = 0.003), MPTA (p = 0.011), and FTFA (p = 0.008) were significant risk factors predicting postoperative valgus malalignment after mobile-bearing UKA.
Conclusions
Preoperative smaller LDFA, FTFA, larger MPTA and less medial tibial cut thickness were significantly associated with postoperative valgus malalignment in mobile-bearing UKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unicompartmental knee arthroplasty (UKA) is a minimal invasive option for anteromedial osteoarthritis with many advantages, such as a smaller incision, less soft-tissue injury, minimal bone resection, preservation of normal knee kinematics, and rapid recovery [1, 2]. UKA has gained popularity in the world since the introduction of minimally invasive surgical technique by Repicci [3,4,5,6].
The progression of osteoarthritis in the lateral compartment is one of the main failure modes of UKA, accounting for approximately 20–40% of UKA failures [7,8,9]. Postoperative valgus malalignment with overcorrection is the most common cause of increased lateral compartment load and leading to osteoarthritis progression [10]. Hernigou et al. evaluated 58 knees UKA with 15-year follow-up and found that an overcorrection in valgus malalignment (HKAA > 180°) was associated with an increased risk of degenerative changes in the lateral compartment [11]. Wen et al. found that 3° valgus would increase load percentage 45.78% of the lateral compartment [12]. Besides, alignment errors may have side effects on the change of knee kinematics, wear rate, implant loosening, or failure [11, 13].
As the exposure is limited in the minimally invasive technique, UKA is generally considered a technical challenge to achieve good postoperative alignment and position. Kim et al. reported 124 Oxford phase 3 UKAs, and 13% cases did not gain acceptable postoperative mechanical axis [14]. Mullaji et al. investigated 122 consecutive minimally invasive Oxford phase 3 medial UKA in 109 patients and found that 11% were in postoperative valgus (HKAA > 180°) [15]. In our clinical practice, some patients also tend to valgus, although most limbs have acceptable alignments after UKA.
Because postoperative valgus alignment may lead to osteoarthritis progression in the lateral compartment, risk factors for postoperative valgus malalignment are important considerations to avoid the complication after medial UKA. However, to the best of our knowledge, few studies have evaluated the risk factors for postoperative valgus malalignment in mobile-bearing medial UKA. Hopgood et al. reported that tibiofemoral angle correction would increase as the thickness of the tibial insert increases [16]. However, it was about the fixed-bearing system which was designed quite different from the mobile-bearing system. The mobile-bearing Oxford UKA was designed to keep knee motion stability by polyethylene insert without soft-tissue release. Therefore, an evaluation of risk factors to predict postoperative alignment in mobile-bearing Oxford UKA may be useful.
The hypothesis of this study was that some preoperative or intraoperative factors would be correlated well with the postoperative valgus malalignment and be useful for predicting postoperative valgus malalignment with mobile-bearing UKA.
Patients and methods
Approval for the present study from the institutional review board was obtained. From January 2016 to December 2017, 122 knees’ consecutive UKAs were included. The indications for UKA were severe knee pain of medial compartment and considerable difficulty in walking and performing daily activities. Radiograph could demonstrate loss of articular cartilage medially by showing that the medial joint width became narrow. The other indications were an intact anterior cruciate ligament (ACL), varus deformity < 15°, flexion contracture < 15°, and intact lateral compartment [17]. The preoperative diagnosis was osteoarthritis in all patients. At baseline, the 122 knees’ medial UKA cohort consisted of 25 knees’ male (20.49%) and 97 females (79.51%), with a mean age of 67.99 ± 8.86 years (49–85 years) and a mean body mass index (BMI) of 26.42 ± 3.26 kg/m2 (18.3–35.2 kg/m2). A total of 56 (45.90%) UKAs were performed on the right knee and 66 (54.10%) on the left knee.
Surgical procedure
All UKA procedures were performed by the senior author using the same minimally invasive surgical technique with the mobile-bearing Oxford medial UKA (Oxford unicompartmental knee, Biomet, Bridgend, UK). The knee joint was exposed through a small incision with quadriceps sparing and no patellar eversion. Medial release for ligament balancing or realignment was not performed. However, all medial osteophytes were completely removed with the osteotome. The ligament balance was determined according to the thickness of the polyethylene insert.
Radiographic assessments
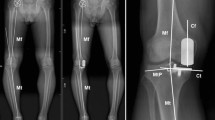
Standardized weight-bearing anteroposterior, lateral, skyline radiographs, and full-length radiographs were obtained at our institution both preoperatively and postoperatively. On full-length weight-bearing radiographs, the hip–knee–ankle angle (HKAA) was measured as the angle between the femoral mechanical axis (center of hip to center of knee) and the tibial mechanical axis (center of knee to center of ankle) [18, 19]. Valgus was defined as HKAA > 180°. The lateral distal femoral angle (LDFA) was measured by the lateral angle between the anatomical axis of the femur and the distal femur articular surface, while the medial proximal tibia angle (MPTA) was defined as the medial angle between the knee joint line of the tibia and the axis line of the tibia. The femorotibial facet angle (FTFA) was defined as the angle between the best-fit line along the surface of the tibial plateau and the line connecting the most distal points of the medial and lateral femur condyles [20] (Fig. 1).
Patients were assessed radiographically on preoperative weight-bearing X-rays. The overall limb alignment hip–knee–ankle angle (HKAA) was defined as the angle among hip center, notch center of distal femur, and ankle talus center. The lateral distal femoral angle (LDFA) was measured by the lateral angle between the distal femur articular surface and the anatomical axis of femur, while medial proximal tibia angle (MPTA) was defined as the medial angle between the knee joint line of tibia and the axis line of tibia
The medial femoral bone mill was recorded as the number of the final spigot used in procedure. The medial tibial bone cut was measured on weight-bearing anteroposterior radiographs using the following method: On preoperative anteroposterior radiograph, both the anatomical axis of the tibia (line A) and a line perpendicular to the anatomical axis from the lowest point of the medial tibia (line B) were drawn. The distance from line B to the peak point of tibial vertices was measured (distance α). On postoperative radiograph, the perpendicular line (line C) to the anatomical axis (line A) from the bottom of tibia implant was drawn. The distance β from the same peak point of tibial vertices to the line C was measured. The difference between distance α and distance β was defined as the medial tibial bone cut thickness (Fig. 2).
Medial tibial bone cut was measured on weight-bearing anteroposterior radiographs: an anatomical axis of the tibia (line A) and a line perpendicular to the anatomical axis from the lowest point of medial tibia (line B) were drawn on the preoperative radiograph. The distance from line B to the peak point of the tibial vertices was measured (distance α). On the postoperative radiograph, the perpendicular line (line C) to the anatomical axis (line A) from the bottom of the tibia implant was drawn. The distance β from the same peak point of the tibial vertices to the lines C was measured. The difference between distance α and distance β was defined as the medial tibial bone cut amount
The component alignments and positions were measured on postoperative radiographs: femoral A—coronal angle of femoral component, femoral B—sagittal angle of femoral component, tibial E—coronal angle of tibial component, and tibial F—posterior–inferior slope of tibial component. Positive values represent varus and flexion alignment, and negative values represent valgus and extension alignment (Fig. 3).
Diagrams showing the postoperative radiographic assessments of component alignment and position: femoral angle A was coronal angle of femoral component; femoral angle B was sagittal angle of femoral component; tibial angle E was coronal angle of tibial component; and tibial angle F was posterior–inferior slope of tibial component. Positive values represent varus and flexion alignment, and negative values represent valgus and extension alignment
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS, Chicago, IL, USA). The data were reported as the mean ± standard deviation. For logistic regression analysis of risk factors of postoperative valgus malalignment, the variables of risk factors included age, gender, BMI, side, preoperative HKAA, preoperative LDFA, MPTA, FTFA, thickness of polyethylene bearing insert, size of tibial prothesis, size of femoral prothesis, thickness of medial tibial bone cut, thickness of distal femoral mill, femoral A, femoral B, tibial E, and tibial F. To identify potential predictors of postoperative valgus malalignment, we compared the 16 variables of group valgus with group non-valgus using a univariate method. Statistical significance of the variables was determined by t test and Chi-square test with p value ≤ 0.1. For the multivariate analysis, the significant risk factors from univariate analysis were selected and a backward stepwise procedure in a multiple logistic regression model was performed with variables entered for p value ≤ 0.1. The results of regression analyses were presented as odds ratio (OR) with 95% confidence intervals (95%CI). The result was considered to be statistically significant when the null value (1.00) was absent from the 95CI or p value < 0.05.
Results
The mean mechanical preoperative HKAA of 174.39°±4.23° was corrected to 178.18°±3.49° postoperatively (t = − 13.45, p = 0.000). The mean of postoperative HKAA in valgus group and non-valgus group was 183.45 ± 2.21° and 176.88 ± 2.35°, respectively (t = 12.44, p = 0.000). There were no differences regarding gender distribution, operating side between groups. After statistical analysis with univariate analysis, eight risk factor variables among 16 independent variables were identified as potential predictors with p value ≤ 0.1. These were age, BMI, preoperative HKAA, preoperative LDFA, MPTA, FTFA, thickness of medial tibial bone cut, and tibial E. Multivariate logistic regression analysis for these eight potential predictors revealed that tibial cut (p = 0.046), LDFA (p = 0.003), MPTA (p = 0.011), and FTFA (p = 0.008) were significant risk factors predicting postoperative valgus malalignment after mobile-bearing UKA (Tables 1, 2).
The mean of tibial cut was 6.59 ± 1.64 mm in valgus group and 7.49 ± 2.41 mm in non-valgus group (p = 0.087). The OR of tibial cut in multivariate analysis was 0.59 (95% CI 0.35–0.99, p = 0.046). As tibial cut decreased by one millimeter, malalignment was about 1.71 times probable.
The mean of preoperative LDFA was 76.53 ± 2.21° in valgus group and 82.06 ± 2.34° in non-valgus group (p = 0.000). In multivariate logistic regression analysis, the OR of preoperative LDFA was 0.18(95% CI 0.06–0.56, p = 0.003). As LDFA decreased by 1º, postoperative valgus malalignment incidence was about 5.44 times increase.
The mean of preoperative MPTA was 87.64 ± 1.91° in valgus group and 85.42 ± 2.61° in non-valgus group (p = 0.000). The OR of preoperative MPTA in multivariate analysis was 5.11 (95% CI 1.46–17.90, p = 0.011). As MPTA increased by 1º, malalignment was about 5.11 times probable.
The mean of preoperative FTFA was 2.56 ± 1.91° in valgus group and 4.06 ± 2.06° in non-valgus group (p = 0.002). In multivariate logistic regression analysis, the OR of preoperative FTFA in was 0.28 (95% CI 0.11–0.72, p = 0.008). As FTFA decreased by 1º, postoperative valgus malalignment was about 3.60 times probable.
Discussion
The most important finding of the present study was that smaller LDFA, FTFA, larger MPTA, and less medial tibial cut thickness were significantly associated with postoperative valgus malalignment in mobile-bearing UKA.
Oxford mobile-bearing UKA is indicated for symptomatic anteromedial osteoarthritis with the design purpose to restore the natural knee motion and minimize polyethylene wear. As surgical techniques and instruments have improved, the procedure has shown good results both in functional outcome and survivorship. Pandit et al. reported 10-year survival rate 99.8% using revision as the end point in 1000 Oxford phase 3 medial UKAs [21]. Lisowski et al. reported that the mean knee society score and function score were all improved after Oxford mobile-bearing UKA with 94.4% 7-year survival rate [22]. Although implant developers and experienced professors reported good results for UKA, many knee replacement registries still reported relatively high failure rates and poor results, especially when compared with total knee arthroplasty (TKA) [23]. Badawy et al. evaluated the data of the Norwegian arthroplasty register from 1999 to 2012 and found that the failure rate was about 20% at 10 years, especially higher in low-volume hospitals [23]. Niinimaki et al. reported that UKA had inferior survivorship compared with TKA in Finnish arthroplasty register, and the Kaplan–Meier survivorship of UKA was only 69.6% at 15 years (TKA 88.7%) [24]. Among many failure models, osteoarthritis progression in lateral compartment almost accounted the top three reasons ranging from 0.9 to 7% [25,26,27,28]. Lewold et al. reported that osteoarthritis progressive was the cause of failure in 25% cases in Swedish knee arthroplasty registry [29]. van der List JP et al. performed a systematic review and reported that osteoarthritis progression was the major failure mode in midterm and late failures (38 and 40%, respectively) [9].
Postoperative alignment in medial UKA has been evaluated by many studies [14, 30,31,32]. Mercier et al. investigated 43 Oxford UKAs with 14.88-year follow-up and found a strong correlation between the postoperative valgus angle and the progression of lateral compartment arthritis (p = 0.005) [33]. Kim et al. found that postoperative tibiofemoral angle ≥ 10° of valgus had the highest failure rate of implants, and the 8-year survival rate was only 69.2% [31]. Xue et al. reported that three lateral progression of osteoarthritis and found a mean postoperative valgus of > 5° at the short time of Oxford medial UKA [34]. Although the ideal postoperative angle is conversational, it is better to avoid postoperative valgus malalignment for UKA.
The limb malalignment deformity in frontal plane includes the intra-articular and extra-articular deformity [35]. The indication of UKA is anteromedial osteoarthritis, whose preoperative varus malalignment is from cartilage erosion in medial compartment. Oxford mobile-bearing UKA just replaces the medial lesion without releasing the ligaments that means that UKA just corrects the intra-articular deformity. The risk factors in this study may indicate the cause of limbs overcorrected to valgus. Smaller LDFA and larger MPTA indicated the physiological habitual valgus. The limb overcorrected to valgus might have a small physiological habitual valgus before developing medial compartmental OA due to medial wear. In these cases, Oxford mobile-bearing UKA corrected the genu varum and restored whatever degree of tibiofemoral valgus present before the arthritis developed. FTFA is the angle between femoral facet and tibial facet, which could reflect the intra-articular deformity [20]. Since a smaller FTFA means less intra-articular deformity, UKA may overstuff the medial compartment when the intra-articular deformity is small. Similarly, less tibial cut cannot create enough gap to insert the prothesis in theory. However, these cases may have loosing medial tissue, so even if the tibial cut is less, the gap is larger enough. Following, overcorrection may incur after UKA [36].
For the effect of the tibial insert thickness on postoperative alignment, we found that postoperative valgus was independent of tibial bearing insert thickness. The result was similar with Ahn JH’s report [37] and quite different with Kim’s study [14], though the latter was about the same Oxford UKA. The Oxford mobile-bearing UKA principle of the procedure was to keep knee stability by ligament tension. Soft-tissue tension must be adequate to prevent the joint from subluxation or dislocation. If UKA aims to keep the natural tension without soft-tissue release, the bearing size inserted is related to ligament relaxation, bone cut, and intra-articular deformity correctability.
The present study had several strengths. This logistic regression analysis was the first study to estimate the risk factors including preoperative radiographic features and surgical characteristics for postoperative valgus malalignment in mobile-bearing UKA. Most previous studies did not focus on the surgical factors, such as tibial cut and femoral mill, which were addressed in the present study. Besides, we introduced simple and concise parameters in the analysis, using LDFA, MPTA for extra-articular deformity and FTFA for intra-articular deformity. Third, it had great value for clinical practice. The result suggested that it might be better to select a patient for mobile-bearing UKA without smaller LDFA, FTFA, and larger MPTA.
Nevertheless, there were still some potential weaknesses in the study. First, the amount of the ligamentous balance was not quantified. However, all procedures were performed by the senior author, and the same surgical criteria of soft tissue and bone preservation were uniform throughout the study. The procedures were achieved in the absence of ligament release, and a standard 1 mm gap was persevered after protheses implant. Second, the sample size of the case series was relatively small. If we included more patients, the result might be more comprehensive. Third, as the aim of this study was to investigate the risk factors of postoperative valgus malalignment, cartilage erosion and excising osteophytes were not analyzed in the study, which might influence the deformity correctability. Further research is still needed to elaborate the result.
Despite these limitations, our study provided valuable information. Preoperative smaller LDFA, FTFA, larger MPTA, and less medial tibial cut thickness were significantly associated with postoperative valgus malalignment in mobile-bearing UKA. Regarding UKA patient selection in practice, patients with the risk factors are not indicated for medial UKA. It might be better to select patients for mobile-bearing UKA without smaller LDFA, FTFA, and larger MPTA. With respect to surgical technique, the amount of medial tibial cut should not be too small to implant a prosthesis.
References
Price AJ, Svard U (2011) A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res 469:174–179
Emerson RH, Alnachoukati O, Barrington J, Ennin K (2016) The results of Oxford unicompartmental knee arthroplasty in the United States: a mean ten-year survival analysis. Bone Jt J 98-B:34–40
Repicci JA, Eberle RW (1999) Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc 8:20–27, 27
Ozcan C, Simsek ME, Tahta M, Akkaya M, Gursoy S, Bozkurt M (2018) Fixed-bearing unicompartmental knee arthroplasty tolerates higher variance in tibial implant rotation than mobile-bearing designs. Arch Orthop Trauma Surg 138:1463–1469
Panzram B, Bertlich I, Reiner T, Walker T, Hagmann S, Gotterbarm T (2017) Cementless Oxford medial unicompartimental knee replacement: an independent series with a 5-year-follow-up. Arch Orthop Trauma Surg 137:1011–1017
Guo WS, Zhang QD, Liu ZH, Cheng LM, Yue DB, Wang WG, Zhang NF, Li ZR (2015) Minimally invasive unicompartmental knee arthroplasty for spontaneous osteonecrosis of the knee. Orthop Surg 7:119–124
Campi S, Pandit HG, Oosthuizen CR (2018) The Oxford Medial Unicompartmental Knee Arthroplasty: The South African Experience. J Arthroplasty 33:1727–1731
Pandit H, Hamilton TW, Jenkins C, Mellon SJ, Dodd CA, Murray DW (2015) The clinical outcome of minimally invasive Phase 3 Oxford unicompartmental knee arthroplasty: a 15-year follow-up of 1000 UKAs. Bone Jt J 97-B:1493–1500
van der List JP, Zuiderbaan HA, Pearle AD (2016) Why do medial unicompartmental knee arthroplasties fail today?. J Arthroplasty 31:1016–1021
Felson DT, Niu J, Gross KD, Englund M, Sharma L, Cooke TD, Guermazi A, Roemer FW, Segal N, Goggins JM, Lewis CE, Eaton C, Nevitt MC (2013) Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum 65:355–362
Hernigou P, Deschamps G (2004) Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res 423:161–165
Wen PF, Guo WS, Gao FQ, Zhang QD, Yue JA, Cheng LM, Zhu GD (2017) Effects of lower limb alignment and tibial component inclination on the biomechanics of lateral compartment in unicompartmental knee arthroplasty. Chin Med J (Engl) 130:2563–2568
Kang KT, Son J, Baek C, Kwon OR, Koh YG (2018) Femoral component alignment in unicompartmental knee arthroplasty leads to biomechanical change in contact stress and collateral ligament force in knee joint. Arch Orthop Trauma Surg 138:563–572
Kim SJ, Bae JH, Lim HC (2012) Factors affecting the postoperative limb alignment and clinical outcome after Oxford unicompartmental knee arthroplasty. J Arthroplasty 27:1210–1215
Mullaji AB, Shetty GM, Kanna R (2011) Postoperative limb alignment and its determinants after minimally invasive Oxford medial unicompartmental knee arthroplasty. J arthroplasty 26:919–925
Hopgood P, Martin CP, Rae PJ (2004) The effect of tibial implant size on post-operative alignment following medial unicompartmental knee replacement. Knee 11:385–388
Murray DW (2005) Mobile bearing unicompartmental knee replacement. Orthopedics 28:985–987
Marx RG, Grimm P, Lillemoe KA, Robertson CM, Ayeni OR, Lyman S, Bogner EA, Pavlov H (2011) Reliability of lower extremity alignment measurement using radiographs and PACS. Knee Surg Sports Traumatol Arthrosc 19:1693–1698
Moreland JR, Bassett LW, Hanker GJ (1987) Radiographic analysis of the axial alignment of the lower extremity. J Bone Jt Surg Am 69:745–749
Zhang Q, Yue J, Wang W, Chen Y, Zhao Q, Guo W (2018) FTFA change under valgus stress force radiography is useful for evaluating the correctability of intra-articular varus deformity in UKA. Arch Orthop Trauma Surg 138:1003–1009
Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW (2011) Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Jt Surg Br 93:198–204
Lisowski LA, van den Bekerom MP, Pilot P, van Dijk CN, Lisowski AE (2011) Oxford Phase 3 unicompartmental knee arthroplasty: medium-term results of a minimally invasive surgical procedure. Knee Surg Sports Traumatol Arthrosc 19:277–284
Badawy M, Espehaug B, Indrekvam K, Havelin LI, Furnes O (2014) Higher revision risk for unicompartmental knee arthroplasty in low-volume hospitals. Acta Orthop 85:342–347
Niinimaki T, Eskelinen A, Makela K, Ohtonen P, Puhto AP, Remes V (2014) Unicompartmental knee arthroplasty survivorship is lower than TKA survivorship: a 27-year Finnish registry study. Clin Orthop Relat Res 472:1496–1501
Epinette JA, Brunschweiler B, Mertl P, Mole D, Cazenave A (2012) Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res 98:S124–S130
Vasso M, Corona K, D’Apolito R, Mazzitelli G, Panni AS (2017) Unicompartmental knee arthroplasty: modes of failure and conversion to total knee arthroplasty. Joints 5:44–50
Weale AE, Murray DW, Crawford R, Psychoyios V, Bonomo A, Howell G, O’Connor J, Goodfellow JW (1999) Does arthritis progress in the retained compartments after ‘Oxford’ medial unicompartmental arthroplasty? A clinical and radiological study with a minimum ten-year follow-up. J Bone Jt Surg Br 81:783–789
Emerson RJ, Higgins LL (2008) Unicompartmental knee arthroplasty with the oxford prosthesis in patients with medial compartment arthritis. J Bone Jt Surg Am 90:118–122
Lewold S, Robertsson O, Knutson K, Lidgren L (1998) Revision of unicompartmental knee arthroplasty: outcome in 1,135 cases from the Swedish Knee Arthroplasty study. Acta Orthop Scand 69:469–474
Mullaji AB, Shah S, Shetty GM (2017) Mobile-bearing medial unicompartmental knee arthroplasty restores limb alignment comparable to that of the unaffected contralateral limb. Acta Orthop 88:70–74
Kim KT, Lee S, Kim TW, Lee JS, Boo KH (2012) The influence of postoperative tibiofemoral alignment on the clinical results of unicompartmental knee arthroplasty. Knee Surg Relat Res 24:85–90
Gulati A, Pandit H, Jenkins C, Chau R, Dodd CA, Murray DW (2009) The effect of leg alignment on the outcome of unicompartmental knee replacement. J Bone Jt Surg Br 91:469–474
Mercier N, Wimsey S, Saragaglia D (2010) Long-term clinical results of the Oxford medial unicompartmental knee arthroplasty. Int Orthop 34:1137–1143
Xue H, Tu Y, Ma T, Wen T, Yang T, Cai M (2017) Up to twelve year follow-up of the Oxford phase three unicompartmental knee replacement in China: seven hundred and eight knees from an independent centre. Int Orthop 41:1571–1577
Maderbacher G, Baier C, Springorum HR, Zeman F, Grifka J, Keshmiri A (2016) Lower limb anatomy and alignment affect natural tibiofemoral knee kinematics: a cadaveric investigation. J Arthroplasty 31:2038–2042
Peersman G, Slane J, Vuylsteke P, Fuchs-Winkelmann S, Dworschak P, Heyse T, Scheys L (2017) Kinematics of mobile-bearing unicompartmental knee arthroplasty compared to native: results from an in vitro study. Arch Orthop Trauma Surg 137:1557–1563
Ahn JH, Kang HW, Yang TY, Lee JY (2016) Risk factors of post-operative malalignment in fixed-bearing medial unicompartmental knee arthroplasty. Int Orthop 40:1455–1463
Acknowledgements
We would like to thank the patients included in the study for their cooperations. We would also like to thank Mr Omar M. Fakhr, Department of Biological Science, The University of Tulsa, Tulsa, OK, 74104, USA, for the English improvement.
Funding
This study was funded by Beijing municipal science and technology commission (Grant number Z171100001017209), National Natural Science Foundation of China (Grant number 81703896), and the Capital Health Research and Development of Special (Grant number 2016-2-4062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent for study participation was obtained from each patient.
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, Q., Guo, W. et al. Risk factors of postoperative valgus malalignment in mobile-bearing medial unicompartmental knee arthroplasty. Arch Orthop Trauma Surg 139, 241–248 (2019). https://doi.org/10.1007/s00402-018-3070-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-3070-2