Abstract
Introduction
The debate on efficacy of fusion added to decompression for lumbar spinal stenosis (LSS) is ongoing. No meta-analysis has compared the effectiveness of decompression versus decompression plus fusion in treating patients with LSS.
Methods
A literature search was performed in the Web of Science, PubMed, Embase, and Springer databases from 1970 to 2016. Relevant references were selected and the included studies were manually reviewed. We included trials evaluating decompression surgery compared to decompression plus fusion surgery in treating patients with LSS. The primary outcomes analyzed were back pain, leg pain, Oswestry Disability Index scores (ODI), the quality-of-life EuroQol-5 Dimensions (EQ-5D), duration of operation, intraoperative blood loss, length of hospital stay, major complications, walking ability, number of reoperation, and finally clinically excellent and good rates. Data analysis was conducted using the Review Manager 5.2 software.
Results
Fifteen studies involving 17,785 patients with LSS were included. The overall effect mean difference (MD) (95% CI) in the differences between pre- and post-operative back pain, leg pain, operative time, intraoperative blood loss, and length of stay were 0.04 (−0.36, 0.44), 0.69 (−0.38, 1.76), −2.04 (−3.12, −0.96), −3.96 (−6.64, −1.27) and −4.21 (−10.03, 1.62) (z = 0.18, 1.26, 3.71, 2.89 and 1.41, respectively; P = 0.86, 0.55, 0.0002, 0.004 and 0.16, respectively) in random effects models. The overall effect MD (95% CI) in ODI, EQ-5D, and walking ability were 0.43 (−1.15, 2.00), 0.01 (−0.01, 0.03) and 0.04 (−0.49, 0.57) (z = 0.52, 1.16 and 0.15, respectively; P = 0.59, 0.24 and 0.88, respectively) in fixed effects models. The overall effect odds ratio (OR) (95% CI) of major complications, number of reoperations, and clinically excellent and good rates between the two groups were 0.70 (0.60, 0.81), 1.04 (0.90, 1.19) and 0.31 (0.06, 1.59) (z = 4.63, 0.53 and 1.40, respectively; P < 0.00001, 0.60 and 0.16, respectively). Our study reveals no difference in the effectiveness between the two surgical techniques.
Conclusions
The additional fusion in the management of LSS yielded no clinical improvements over decompression alone within a 2-year follow-up period. But fusion resulted in a longer duration of operation, more blood loss, and a higher risk of complications. Therefore, the appropriate surgical protocol for LSS should be discussed further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar spinal stenosis (LSS) is characterized by narrowing of the central vertebral canal, lateral recesses, and vertebral foramina. LSS causes patients significant long-term symptoms (e.g., intermittent neurogenic claudication, radicular back and leg pain), and patients often do not respond well to conservative treatments such as physiotherapy, analgesics, and steroids [1–4]. Decompression surgery can achieve neural compromise and improve the associated pain by relieving lumbar canal stricture and removing redundant tissue. Laminectomy, as one technique of decompression surgery, is a recommended surgical approach for LSS [5]. Unilateral laminotomy with bilateral decompression (ULBD), a less invasive surgical technique proposed by Chang et al. was demonstrated to immediately and substantially improve the physical score and bodily pain score in patients with LSS [6]. However, many post-operative complications and other lumbar degenerative diseases, especially spondylolisthesis, were associated with decompression. Mardjetko et al. reported a high incidence (31%) of slip progression in laminectomy alone for LSS with degenerative spondylolisthesis (DS) [7].
Fusion can more appropriately treat the defect of progressive lumbar instability after decompression. In the last few decades, decompression plus fusion had become very popular and was regarded as a gold standard for LSS. However, the debate over its use was never settled, due to spinal fusion being a more traumatic procedure, requiring a longer operative time, causing more blood loss, and exhibiting an increased complication rate in extremely elderly patients [8–11]. Many authors reported similar treatment outcomes between decompression alone and decompression plus fusion among the majority of patients with LSS and DS, and they sought to uphold the view of there being no absolute need for additional fusion [12–16].
Because there is a paucity of evidence—particularly in primary evidence—supporting either argument, we conducted a meta-analysis aiming to compare the efficacy of decompression alone versus decompression plus fusion for LSS. We also discussed the optimal indications for performing additional surgical fusions to treat this condition.
Methods
Literature search and evaluation
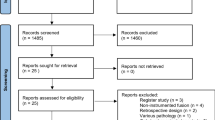
An online search was performed in the Web of Science, PubMed, Embase, and Springer databases, from January 1970 to May 2016. Relevant references were selected and the included studies were manually reviewed. The search strategy is detailed in Fig. 1. To facilitate future updates of this systematic review and meta-analysis, we present the search strategy as follows: (laminotomy OR laminectomy OR fenestration OR hemilaminectomy OR decompression) AND (lumbar spondylolisthesis OR lumbar spinal stenosis OR lumbar canal stenosis OR degenerative lumbar spondylolisthesis) AND (fusion OR arthrodesis).
Eligibility criteria
Included studies fulfilled the following criteria: (1) they were randomized controlled trials (RCTs) or clinical cohort studies written in English; (2) the studies assessed the comparison between decompression alone versus fusion plus decompression surgery for LSS; (3) the LSS was with or without grade I DS; and (4) the studies reported the means and standard deviations of intra- and post-operative assessments with sample size between decompression alone group and decompression plus fusion group, as well as the reported number and total number of major complications, reoperations, and clinically excellent and good rates between the two groups [or alternately the studies provided sufficient data to construct those contingency tables (the difference between pre- and post-operative back and leg pain, EQ-5D, and ODI in Försth et al. [40] and Ghogawala et al. [17])] were constructed by Graphpad instat 3.0, which was developed by GraphPad software company in America.
Exclusion criteria
Studies were excluded if they were: (1) non-English-language articles, case reports, duplicate papers, or conference reports; (2) original articles without a controlled group or with a small sample size (<16 patients); (3) studies investigating mixed lumbar degenerative diseases (LDD), tumors, fractures, osteoporosis, or other non-degenerative diseases; (4) studies not specifically concerning decompression alone and fusion plus decompression surgery; (5) studies mainly evaluating a surgical approach, or new/non-standardized surgical techniques or instruments; or, finally, (6) studies with incomplete or unacceptable information for a comparison.
Data collection and methodological quality
Two authors independently sorted and reviewed all abstracts or full texts of the retrieved articles based on eligibility and exclusion criteria. Disagreements were resolved by consensus between two senior orthopedics. Data were extracted and summarized as follows: (1) the basic characteristics of each study: author, publication year, country, age, sex ratio, research period, comorbidities, surgery type, and follow-ups (within a 2-year period) were reported; (2) the primary outcomes of back pain and leg pain (when comparison was shown between back and leg pain, BP > LP were selected), walking ability, major quality-of-life EuroQol-5 Dimensions (EQ-5D), Oswestry Disability Index (ODI) scores, and Japanese Orthopaedic Association (JOA) scores (as many articles did not offer total scores in these questionnaires, partial sub-items that met our measures were abstracted instead) were reported; (3) secondary outcomes that included intraoperative surgical data (blood loss and duration of operation) and patient-reported outcomes (length of stay, complications, reoperations, and clinical satisfaction) were reported. All studies were assessed for quality evaluation according to the classic Newcastle–Ottawa Scale (NOS). Scale scores range from zero to nine points, with higher scores indicating better quality.
Data analysis
Statistical analyses were performed using the Cochrane Collaboration’s Review Manager 5.2 software. Pooled weighted mean differences (WMDs) with 95% confidence intervals (CIs) for continuous variables and pooled odds ratios (ORs) with 95% CIs for enumeration data were calculated. A Z test was performed to determine the overall effects. If the heterogeneity between studies was statistically significant (I 2 ≥ 50%), a random effects model was used for further sensitivity analysis. Otherwise, a fixed effects model was selected (I 2 < 50%). Influential analysis was examined by removing one individual study at one time to check heterogeneity that biased the overall estimate. Two-sided P ≤ 0.05 was considered statistically significant.
Results
A total of 4442 studies in the Web of Science (1063), PubMed (1643), Embase (732), and Springer (1004) databases were reviewed. After perusing the titles and abstracts, 53 relevant studies were identified, and, with further meticulous examination, 15 studies were finally selected that met our eligibility criteria and were transferred to data abstraction for statistical analysis (Fig. 1). A description of the main characteristics of all studies is listed in Table 1.
Results of meta-analysis
Basic characteristics
A total of 17,785 cases in 15 articles were finally enrolled in our study, including those from five controlled trials. Overall, 12,417 patients with LSS received decompression surgery alone, compared to 5368 patients who underwent decompression plus fusion surgery. The number of females and males was available in 12 studies containing 6549 females and 3187 males, with a sex ratio (F/M) of 2.05. Overall, the average age of patients with LSS could not be calculated, because most studies could be separately described according to sex or surgical strategy. While the likely dominant age was approximately 65 years, females were higher in numbers and average onset age than males.
Primary measures
Back pain
Eight studies reported changes in back pain between the two subgroups, including two based on JOA and six based on VAS. With regard to the overall results of heterogeneity testing, there was a statistically significant difference between the two groups (P = 0.003, I 2 = 67%), and a random effects model was applied for meta-analysis (Fig. 2). No statistically significant difference was found in the changes between pre- and post-operative back pain evaluated according to JOA and VAS between the two groups [JOA subgroup, MD = 0.01, 95% CI (–0.41, 0.42), z = 0.03, P = 0.98; VAS subgroup, MD = 0.01, 95% CI (–0.52, 0.54), z = 0.04, P = 0.97].
Leg pain
Seven studies reported changes in leg pain between the two subgroups, including six based on VAS and one according to JOA. A heterogeneity test indicated a statistically significant difference between the two groups (P < 0.00001, I 2 = 96%), and a random effects model was applied for meta-analysis (Fig. 3). In the VAS subgroup, MD = 0.79, 95% CI (–0.47, 2.05), z = 1.23, and P = 0.22; in the JOA subgroup, MD = 0.10, 95% CI (–0.23, 0.43), z = 0.59, and P = 0.55. These results demonstrated that the differences in pre- and post-operative leg pain were not significantly different between the two groups.
ODI
Five studies reported ODI in the two groups. The heterogeneity test showed no statistically significant difference between the two groups (P = 0.25, I 2 = 25%), and a fixed effects model was applied for meta-analysis (Fig. 4). There was no significant difference in ODI between the decompression group and the decompression plus fusion group (MD = 0.43, 95% CI (–1.15, 2.00), z = 0.53, P = 0.59).
EQ-5D
Two studies reported EQ-5D in the two groups. There was no statistically significant heterogeneity between the two groups (P = 0.34, I 2 = 0%), and a fixed effects model was applied for meta-analysis (Fig. 5). No statistically significant difference was identified between the two groups.
Duration of operation
Three studies reported the duration of operation in the two groups. There was statistically significant heterogeneity between the two groups (P < 0.00001, I 2 = 96%). A random effects model was applied for meta-analysis (Fig. 6), which indicated that the decompression plus fusion group underwent more operative time than the decompression alone group.
Intraoperative blood loss
Three studies reported intraoperative blood loss in the two groups. Statistically significant heterogeneity was found between the two groups (P < 0.00001, I 2 = 97%). A random effects model was applied for meta-analysis (Fig. 7), which demonstrated that the blood loss in the decompression alone group was significantly less than in the decompression plus fusion group.
Length of hospital stay
Two studies reported the length of hospital stay in the two groups. There was statistically significant heterogeneity between the two groups (P = 0.006, I 2 = 87%), and a random effects model was applied for meta-analysis (Fig. 8). The length of hospital stay was not found to be statistically different between the decompression alone group and decompression plus fusion group.
Major complications
Five studies reported major complications in the two groups with a heterogeneity test showing relatively lower statistically significant heterogeneity between groups (P = 0.30, I 2 = 18%). A fixed effects model was applied for meta-analysis (Fig. 9), which indicated that the decompression plus fusion group has approximately 1.4 times higher risk of sustaining major complications than the decompression alone group.
Walking ability
Two studies reported walking ability evaluated according to JOA in the two groups. No statistically significant heterogeneity was found between the two groups (P = 0.58, I 2 = 0%), and a random effects model was applied for meta-analysis (Fig. 10). The walking ability recovery among patients receiving decompression plus fusion surgery was not better than those in the decompression alone group.
Number of reoperation
Three studies reported reoperation numbers in the two groups. Extremely low heterogeneity was identified between the two groups (P = 0.48, I 2 = 0%), and a fixed effects model was applied for meta-analysis (Fig. 11). There was no statistically significant difference between the two groups [OR = 1.04, 95% CI (0.90, 1.19), z = 0.52, P = 0.60].
Clinically excellent and good rates
Four studies reported the clinically excellent and good rates in the two groups. A significantly different heterogeneity was found between the two groups (P = 0.010, I 2 = 74%), and a random effects model was applied for meta-analysis (Fig. 12). The clinical satisfaction in the decompression plus fusion group was not better than in the decompression alone group [OR = 0.31, 95% CI (0.06, 1.59), z = 1.40, P = 0.16].
Sensitivity analysis
When the random effects models were applied, the overall effect MD (95% CI) of the difference in pre- and post-operative back pain and leg pain, operative time, intraoperative blood loss, and length of hospital stay were 0.04 (–0.36, 0.44), 0.69 (−0.38, 1.76), −2.04 (−3.12, −0.96), −3.96 (−6.64, −1.27) and −4.21 (−10.03, 1.62) (z = 0.18, 1.26, 3.71, 2.92 and 1.41, respectively; P = 0.86, 0.55, 0.0002, 0.004 and 0.16, respectively). When the fixed effects models were applied, the overall effect MD (95% CI) of ODI, EQ-5D and walking ability were 0.43 (−1.15, 2.00), 0.01 (−0.01, 0.03) and 0.04 (−0.49, 0.57) (z = 0.52, 1.16 and 0.15, respectively; P = 0.59, 0.24 and 0.88, respectively). The overall effect OR (95% CI) of major complications, number of reoperations, and clinically excellent and good rates between the two groups were 0.70 (0.60, 0.81), 1.04 (0.90, 1.19) and 0.31 (0.06, 1.59) (z = 4.63, 0.53 and 1.40, respectively; P < 0.00001, 0.60 and 0.16, respectively). Except for blood loss, the residual results were consistent between the random and fixed effects models, suggesting that our findings were reliable.
Publication bias
Considering the small sample size (<10) in our meta-analysis, funnel plot analysis was not applicable for publication bias.
Discussion
The limited number of included publications, the relatively low quality, and the inconsistent descriptions of some measures in the 15 selected studies greatly affected the quality of our meta-analysis. Also, the converted parameters possibly impaired the stability of our final outcomes. In our meta-analysis, there was no difference in the effectiveness between decompression alone versus decompression plus fusion; however, patients in the decompression plus fusion group lost more blood, experienced prolonged operation time, and suffered more major complications.
Recently, two published RCTs concentrated on whether decompression plus fusion surgery yielded better clinical results than decompression alone for LSS. In the study by Zoher Ghogawala et al., a slight improvement in overall physical health-related quality-of-life was observed with spinal fusion and laminectomy for LSS, with or without grade I DS [17]. Forsth et al. then concluded that patients with LSS who underwent fusion plus decompression surgery received no better clinical outcomes than patients who received single decompression surgery at long-term follow-ups [18]. Carreon et al. [8], Glassman et al. [9] and Cassinelli et al. [10] demonstrated that posterior spinal fusion following decompression led to longer operative time, more blood loss, and a higher complication rate in extremely elderly patients. To our knowledge, no meta-analysis has compared the effectiveness of decompression versus decompression plus fusion in patients with LSS.
The debate on efficacy of additional fusion for LSS is ongoing. Decompression without arthrodesis was recommended for typical LSS with no history of previous lumbar spine operation, no spinal instability, and DLS ≤20 [19]. Decompression alone has been demonstrated to be significantly less invasive than decompression combined with spinal fusion [20]. Although decompression seems to be the logical procedure that has the potential to give the patient immediate relief, instability of the spine is a potential consequence that needs to be considered [21, 22]. Yone et al. reported that decompression alone for LSS obtained good results only if patients did not present with concomitant spinal instability; otherwise, isolated decompression surgery cannot guarantee satisfactory clinical outcomes [23]. Herkowitz et al. reported that one-third of patients receiving isolated decompression were not satisfied with the outcomes, especially in cases presenting with concomitant lumbar instability, which might lead to a higher reoperation rate [24].
Spinal fusion was initially used by Harms and Rolinger [25]. Suk et al. reported that spinal fusion after a complete decompression can alleviate future back and leg pain [26]. Kleinstueck et al. and Martin et al. both found better results when fusion was added to decompression in patients with LSS and DS [27, 28]. However, as an invasive procedure, fusion has many uncertainties that can greatly influence the final outcomes of LSS. The altered biomechanical function of the spine, such as loss of motion at the fused levels, was compensated for by increased motion at the unfused segments. This process caused certain mechanical stresses, which then accelerated adjacent lumbar level fusion problems and produced back pain and leg pain [26, 29]. Ekman et al. also confirmed this pathological change in a long-term RCT [30]. However, the dilemma of fusion was also inherent in the surgical indication for LSS. Patients with long-standing preoperative symptoms and concomitant diseases often had poor results and less satisfaction in clinical outcomes [15]. Mixed lumbar degenerative diseases and other degenerative changes, such as osteophyte formation, decreased disc height, and calcified ligaments, strengthened the lumbar stability and thereby reduced the demand of fusion. The most important disadvantage of fusion was the co-existence of other complications, higher reoperation rates, and heavier financial costs. Hallett et al. revealed a cost difference of approximately USD $6290 per patient for an additional fusion implant [31]. Dailey et al. [32] suggested that neither technique (lumbar fusion or instrumentation and nonfusion) was associated with an increased reoperation rate at the surgical level or adjacent lumbar levels. He noted that the only specific risk factor for reoperation between lumbar fusion and nonfusion was a duration of pretreatment symptoms of more than 12 months (i.e., the natural history of spinal degenerative disease). He also reported a 13% reoperation rate, similar to the 8.0% in our meta-analysis. Brodke et al. summarized that the common reason for reoperation in patients treated with laminectomy and fusion was due to symptomatic adjacent segment pathology; he also found that additional fusion had no superior survival curve, improved clinical outcomes, or improved patient satisfaction rates over laminectomy alone [33].
Therefore, surgeons should exercise great caution while performing spinal fusion in patients with LSS. A stratification of carefully screened patients on the basis of age, gender, comorbidities, with or without preoperative spondylolisthesis, intraoperative evaluation of slippage possibility, and other considerations should be completed, and the ultimate goal of treating LSS need always focus on the balance between decompression of the compressed nerve and adequate bone retention for spinal mechanical stability [16]. McCullen proposed that women and patients with preoperative spondylolisthesis may require changes in decompression without fusion modality to improve outcomes or alterations in long-term expectations for LSS [34]. Brown et al. affirmed intraoperative spinal stiffness measurements did not predict clinical results after lumbar spine surgery [35].
The proper indications for fusion remained unclear, but after searching the literature, some authors’ personal experiences may offer us some guidelines. Matsudaira reported better clinical results in patients with grade IDS by preserving the posterior elements of the spinal canal roof [36]. Radcliff recommended decompression and spinoplasty to preserve posterior ligament complex integrity for multilevel lumbar canal stenosis [37]. Yone suggested Posner’s method to define instability for fusion treatment, and fusion with instrumentation should be performed on elderly patients with instability after decompression [23]. Lawhorne preferred artificial ligamentous bands to decrease the flexion instability [29]. Tuli et al. concluded that the best alternative was an adequate decompressive laminectomy with a nonfusion technique of preserving the posterior ligament complex integrity [38].
The limitations of our study were: (1) the relatively low quality of the 15 included trials, which used multimodal decompression types as well as fusion treatments and made the results less effective; (2) insufficient data in EQ-5D, walking ability, and length of stay; (3) the various complications and nonconformity of assessment criteria in clinical satisfaction, which shared some inner inconsistencies that may have contributed to risk bias; (4) useful objective indicators such as cost-utility, post-operative walking distance, and SF-36, for example, were lacking; (5) our study follow-up period was less than 2 years. A longer-term analysis, including more comparative trials with moderate and high grade evidence, would be expected to improve the validity and reliability of our outcome.
Conclusions
Decompression plus fusion yielded no better clinical results than decompression alone in treating LSS, while resulting in a longer duration of operation, more blood loss, and a higher risk of complications. We believe decompression alone to be a sound choice for LSS, and we expect more controlled trials, prospective studies, and multi-center studies to further testify the long-term outcomes of additional fusion. More research is required to delineate the precise surgical protocol for LSS.
Abbreviations
- JOA score:
-
Japanese Orthopaedic Association scoring system
- LSS:
-
Lumbar spinal stenosis
- DS:
-
Degenerative spondylolisthesis
- MD:
-
Mean difference
- OR:
-
Odd ratio
- LDD:
-
Lumbar degenerative diseases
- ODI:
-
Oswestry Disability Index scores
- EQ-5D:
-
The quality-of-life EuroQol-5 Dimensions
- RCT:
-
Randomized, controlled trial
- VAS:
-
Visual analogue score
- NOS:
-
The classic Newcastle–Ottawa Scale
- ULBD:
-
Unilateral laminotomy with bilateral decompression
- LBP:
-
Low back pain
- DDD:
-
Degenerative disc disease
References
Verbiest H (1955) Further experiences on the pathological influence of a developmental narrowness of the bony lumbar vertebral canal. J Bone Jt Surg Br 37:576–583
Wiltse LL, Kirkaldy-Willis W, McIvor G (1976) The treatment of spinal stenosis. Clin Orthop Relat Res 115:83–91
Verbiest H (1977) Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of twenty-seven years’ experience. J Bone Jt Surg Br 59:181–188
Grabias S (1980) Current concepts review. The treatment of spinal stenosis. J Bone Jt Surg Am 62:308–313
Machado GC, Ferreira PH, Harris IA et al (2015) Effectiveness of surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS One 10:e0122800
Chang HS, Fujisawa N, Tsuchiya T et al (2014) Degenerative spondylolisthesis does not affect the outcome of unilateral laminotomy with bilateral decompression in patients with lumbar stenosis. Spine 39:400–408
Mardjetko S, Connolly P, Shott S (1994) Degenerative lumbar spondylolisthesis: a meta-analysis of literature 1970–1993. Spine 19:2256–2265
Carreon LY, Puno RM, Dimar JR 2nd et al (2003) Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Jt Surg Am 85:2089–2092
Glassman SD, Carreon LY, Dimar JR et al (2007) Clinical outcomes in older patients after posterolateral lumbar fusion. Spine J 7:547–551
Cassinelli EH, Eubanks J, Vogt M et al (2007) Risk factors for the development of perioperative complications in elderly patients undergoing lumbar decompression and arthrodesis for spinal stenosis: an analysis of 166 patients. Spine 32:230–235
Raffo CS, Lauerman WC (2006) Predicting morbidity and mortality of lumbar spine arthrodesis in patients in their ninth decade. Spine 31:99–103
Nasca RJ (1989) Rationale for spinal fusion in lumbar spinal stenosis. Spine 14:451–454
Johnsson K-E, Redlund-Johnell I, Uden A et al (1989) Preoperative and postoperative instability in lumbar spinal stenosis. Spine 14:591–593
Getty C, Kirwan E, Sullivan M (1981) Partial undercutting facetectomy for bony entrapment of the lumbar nerve root. J Bone Jt Surg Br 63:330–335
Gelalis ID, Arnaoutoglou C, Christoforou G et al (2010) Prospective analysis of surgical outcomes in patients undergoing decompressive laminectomy and posterior instrumentation for degenerative lumbar spinal stenosis. Acta Orthop Traumatol Turc 44:235–240
Grobler LJ, Robertson PA, Novotny JE et al (1993) Decompression for degenerative spondylolisthesis and spinal stenosis at L4–5: the effects on facet joint morphology. Spine 18:1475–1482
Ghogawala Z, Dziura J, Butler WE et al (2016) Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 374:1424–1434
Forsth P, Olafsson G, Carlsson T et al (2016) A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med 374:1413–1423
Hansraj KK, O’Leary PF, Cammisa FP Jr et al (2001) Decompression, fusion, and instrumentation surgery for complex lumbar spinal stenosis. Clin Orthop Relat Res 384:18–25
Transfeldt EE, Topp R, Mehbod AA et al (2010) Surgical outcomes of decompression, decompression with limited fusion, and decompression with full curve fusion for degenerative scoliosis with radiculopathy. Spine 35:1872–1875
Amundsen T, Weber H, Nordal HJ et al (2000) Lumbar spinal stenosis: conservative or surgical management?: a prospective 10-year study. Spine 25:1424–1436
Burgstaller JM, Porchet F, Steurer J et al (2015) Arguments for the choice of surgical treatments in patients with lumbar spinal stenosis—a systematic appraisal of randomized controlled trials. BMC Musculoskelet Disord 16:1
Yone K, Sakou T, Kawauchi Y et al (1996) Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine 21:242–248
Herkowitz H, Kurz L (1991) Degenerative lumbar spondylolisthesis with spinal stenosis. J Bone Jt Surg 73:802–808
Harms J, Rolinger H (1981) A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion. Z Orthop Ihre Grenzgeb 120:343–347
Suk S-I, Lee C-K, Kim W-J et al (1997) Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine 22:210–219
Kleinstueck F, Fekete T, Mannion A et al (2012) To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J 21:268–275
Martin CR, Gruszczynski AT, Braunsfurth HA et al (2007) The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine 32:1791–1798
Lawhorne TW III, Girardi FP, Mina CA et al (2009) Treatment of degenerative spondylolisthesis: potential impact of dynamic stabilization based on imaging analysis. Eur Spine J 18:815–822
Ekman P, Moller H, Shalabi A et al (2009) A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J 18:1175–1186
Hallett A, Huntley JS, Gibson JN (2007) Foraminal stenosis and single-level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine 32:1375–1380
Dailey A, Harrop JS, France JC (2010) High-energy contact sports and cervical spine neuropraxia injuries: what are the criteria for return to participation? Spine 35:S193–S201
Brodke DS, Annis P, Lawrence BD et al (2013) Reoperation and revision rates of 3 surgical treatment methods for lumbar stenosis associated with degenerative scoliosis and spondylolisthesis. Spine 38:2287–2294
McCullen GM, Bernini PM, Bernstein SH et al (1994) Clinical and roentgenographic results of decompression for lumbar spinal stenosis. J Spinal Disord 7:380–387
Brown MD, Wehman KF, Heiner AD (2002) The clinical usefulness of intraoperative spinal stiffness measurements. Spine 27:959–961
Matsudaira K, Yamazaki T, Seichi A et al (2005) Spinal stenosis in grade I degenerative lumbar spondylolisthesis: a comparative study of outcomes following laminoplasty and laminectomy with instrumented spinal fusion. J Orthop Sci 10:270–276
Radcliff K, Curry P, Hilibrand A et al (2013) Risk for adjacent segment and same segment reoperation after surgery for lumbar stenosis: a subgroup analysis of the Spine Patient Outcomes Research Trial (SPORT). Spine 38:531
Tuli SM, Kapoor V, Jain AK et al (2011) Spinaplasty following lumbar laminectomy for multilevel lumbar spinal stenosis to prevent iatrogenic instability. Indian J Orthop 45:396–403
Munting E, Roder C, Sobottke R et al (2015) Patient outcomes after laminotomy, hemilaminectomy, laminectomy and laminectomy with instrumented fusion for spinal canal stenosis: a propensity score-based study from the Spine Tango registry. Eur Spine J 24:358–368
Försth P, Michaëlsson K, Sandén B (2013) Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis? A two-year follow-up study involving 5390 patients. Bone Jt J 95:960–965
Kleinstueck FS, Fekete TF, Mannion AF et al (2012) To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J 21:268–275
Sigmundsson FG, Jonsson B, Stromqvist B (2015) Outcome of decompression with and without fusion in spinal stenosis with degenerative spondylolisthesis in relation to preoperative pain pattern: a register study of 1624 patients. Spine J 15:638–646
Sigmundsson FG, Jonsson B, Stromqvist B (2014) Preoperative pain pattern predicts surgical outcome more than type of surgery in patients with central spinal stenosis without concomitant spondylolisthesis: a register study of 9051 patients. Spine 39:E199–E210
Aihara T, Toyone T, Aoki Y et al (2012) Surgical management of degenerative lumbar spondylolisthesis: a comparative study of outcomes following decompression with fusion and microendoscopic decompression. J Musculoskelet Res 15:1250020
Hu RW, Jaglal S, Axcell T et al (1997) A population-based study of reoperations after back surgery. Spine 22:2265–2270
Lad SP, Babu R, Ugiliweneza B et al (2014) Surgery for spinal stenosis: long-term reoperation rates, health care cost, and impact of instrumentation. Spine 39(12):978–987
Grob D, Humke T, Dvorak J (1995) Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Jt Surg Am 77:1036–1041
Postacchini F, Cinotti G (1992) Bone regrowth after surgical decompression for lumbar spinal stenosis. J Bone Jt Surg Br 74:862–869
Kim S, Mortaz Hedjri S, Coyte PC et al (2012) Cost-utility of lumbar decompression with or without fusion for patients with symptomatic degenerative lumbar spondylolisthesis. Spine J 12:44–54
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
W. Chang and P. Yuwen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chang, W., Yuwen, P., Zhu, Y. et al. Effectiveness of decompression alone versus decompression plus fusion for lumbar spinal stenosis: a systematic review and meta-analysis. Arch Orthop Trauma Surg 137, 637–650 (2017). https://doi.org/10.1007/s00402-017-2685-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2685-z