Abstract
Introduction
Besides CRP in serum, white cell counts and cultures of synovial fluid are routinely used to detect periprosthetic joint infections. But the sensitivities of these parameters do vary from 12 to 100 %. In two stage revision arthroplasty before the second stage surgeons have to decide if reimplantation is justified. Therefore, we investigated the value of cultures and white cell count from the synovial fluid with a polymethyl methacrylate spacer in place and CRP in serum before reimplantation to detect persistent infection in a standardized setting.
Methods
115 patients with a two-stage revision hip or knee arthroplasty were included in this study. All patients had an antibiotic loaded polymethylmethacrylate spacer. Retrospectively synovial cultures, white blood count in synovial fluid and CRP in serum were assessed before reimplantation.
Results
The sensitivity of the synovial cultures was 5 % (95 % CI 0.13–24.87), with a specificity of 99 % (95 % CI 94.27–99.97). For white blood count in synovial fluid the sensitivity was 31.3 %, specificity was 39.1 %. Sensitivity for CRP in serum was 42.10 %, specificity was 84.21 %.
Conclusion
Cultures from synovial fluid and white blood count in synovial fluid and CRP seem to be uncertain parameters to exclude persistent infection. We do not recommend joint aspiration before reimplantation anymore. Further research is necessary to find other markers to confirm or exclude persistent infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periprosthetic joint infection (PJI) is still one of the most severe complications in total hip and knee arthroplasty. In the annual report of the Swedish Hip Arthroplasty Register of 2013 already 13.5 % of the revisions were deep infections, whereas in 2000 infection rates of 7.5 % are described in the literature [1, 2]. CRP in serum, white blood counts and cultures of synovial fluid are routinely used to detect PJI. But the sensitivities of these parameters do vary from 12 to 100 % and in 4 % CRP in serum is even negative [3, 4].
Reasons for this poor accuracy can be various: an antibiotic free interval of less than 14 days, cultures were incubated less than 10–14 days and the fact that bacteria are present in a biofilm form with adherence to foreign bodies [5, 6]. In two-stage revision arthroplasty implantation of an antibiotic loaded bone cement spacer have become the Gold standard of treating PJI, although complications such as dislocation and fracture of the spacer need to be taken into account. The advantage of this approach is the local release of antibiotics [7–9]. But still reinfection rates vary from 4 to 50 % [10–17].
Therefore we asked: Is it useful to take cultures from the synovial fluid with a PMMA spacer in place before reimplantation to detect persistent infection and what is the value of CRP in serum and white blood count in synovial fluid before reimplantation in a standardized setting?
Patients and methods
In accordance to the International Consensus on Periprosthetic Joint Infection from 2013, we only included patients with the major criterion of identical organism in at least two periprosthetic cultures out of at least three tissue samples [18, 19].
Between 2008 and 2010 115 patients (44 men, 71 women), with a mean age of 70 years (range 43–92 years) were retrospectively included. 59 patients had periprosthetic joint infections of the knee and 56 of the hip. The indications for total hip arthroplasty (THA) and total knee arthroplasty (TKA) were osteoarthritis n = 91, post traumatic arthritis n = 11, rheumatoid arthritis n = 5.
The infections were defined in all cases as delayed (between the 3rd and 24th month) or late chronic (over 2 years after implantation) [20].
Synovial fluid and tissue samples were incubated up to 14 days. White blood count in synovial fluid was carried out using flow cytometry. All patients were treated with a handmade antibiotic loaded bone cement spacer. Routinely we used Refobacin® Revision bone cement from Biomet, Warsawa, Indiana with 1 g gentamycin and 1 g clindamycin/40 g of cement. A fixed spacer was used in patients with TKA infection, and a mobile spacer in patients with THA infection. After explantation, the patients received intravenous antibiotics for 14 days, followed by oral antibiotic treatment for 4 weeks. All antibiotic treatments were done in cooperation with our institute of microbiology. In ten patients a revision after the first stage because of wound healing problems or because of the resistance of the found bacteria against the antibiotic loaded spacer had to be performed within 14 days after the first stage. In these ten cases the spacer was exchanged and in one of these additional 2 g vancomycin were mixed to the spacer as an off label application.
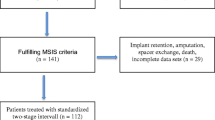
After a 14 days pause in antibiotic treatment, synovial fluid with the spacer in place was aspirated under fluoroscopy and sterile circumstances for cultures and white blood counts. CRP in serum was also assessed. Replantation was carried out in 104 cases and a spacer exchange in 11 cases 10–14 weeks after explantation. Spacer exchanges were carried out if the CRP was not declining or even rising in the postoperative course, a new sinus track and/or signs of local inflammation after the withdrawal of antibiotic treatment were present or if the culture of the synovial fluid before the second stage was positive. Other sources of infection were excluded. In Fig. 1 the progress of all patients is shown.
The results of the intraoperative tissue samples (at least three) during second stage revision (115 pat.) were evaluated in comparison with the results of preoperative aspiration with the spacer in place and with the laboratory parameters. Again in accordance to the International Consensus on Periprosthetic Joint Infection from 2013 at least two identical positive tissue samples during the second stage revision were evaluated as persistent infection.
Statistical analyses
Statistical analyses were performed using IBM-SPSS Statistics v. 22 (IBM, Armonk, NY). The distributions of non-normally distributed parameters between infectious and non-infectious cases were compared using the non-parametric Mann–Whitney U test. p values were regarded as statistically significant if the p value was ≤0.05. Receiver operating characteristic (ROC) analyses were performed to evaluate the diagnostic performance of each marker. The diagonal line in the ROC diagram corresponds to a random guess in the diagnosis of infection. The area under the diagonal line is equal to 0.5. We calculated the area under the ROC curve (AUC) with 95 % confidence intervals (CI). This represents the probability that the marker value of a randomly chosen infectious case ranks higher than the marker value of a randomly chosen noninfectious case. Threshold values for optimal diagnosis of infection were determined using classification and regression tree (CART) analyses. Using the determined cut-off values, the diagnostic performance of the respective marker was evaluated as follows: the sensitivity is the proportion of cases with elevated results among all infectious cases; the specificity is the proportion of cases with values which were not elevated among all noninfectious cases; the positive predictive value (PPV) is the proportion of infectious cases among all cases with elevated results; the negative predictive value (NPV) is the proportion of non-infectious cases among all cases with no elevation of their results.
The study was approved by local ethics committee.
Results
The results of the cultures with the spacer in place are shown in Table 1. Two cultures were positive (1.74 %). One positive culture was confirmed by the intraoperative culture during second stage revision.
So the sensitivity of the aspiration results before replantation was 5 % (95 % CI 0.13–24.87) and the specificity was 99 % (95 % CI 94.27–99.97). The negative predictive value (NPV) was 83 % and the positive predictive value (PPV) was 50 %.
Table 2 shows the values for CRP in serum and white blood counts in synovial fluid before the second-stage revision in cases of positive and negative cultures during second-stage revision.
There were no significant differences in the parameters between positive and negative cultures at the second stage revision. The area under the curve (AUC) for CRP in serum was 0.625 [±0.074 (95 % CI 0.48–0.771)]. The cut-off-value for CRP was 2.3 mg/dl, sensitivity was 42.10 %, and specificity was 84.21 %. The positive predictive value was 34.78 %, the negative predictive value was 87.91 %.
For white blood count in synovial fluid the AUC was 0.371 [±0.09 (95 % CI 0.194–0.548)]. The cut-off-value for white blood count in synovial fluid was 970/µl, sensitivity was 31.3 %, and specificity was 39.1 %. The positive predictive value was 10.6 %, the negative predictive value was 71.1 %. During second stage revision again we took at least three tissue samples [14, 15]. In 20 cases (17.4 %) two tissue cultures out of at least three were positive for bacteria. The bacteria changed between explantation and replantation in 15 cases (75 %), same bacteria were identified in five cases. All patients were put on i. v. antibiotics for 14 days. After a follow up of 24 months we analyzed all patients with two positive cultures during replantation (n = 19) regarding another revision because of a recurrent PJI. In five patients (26.3 %) the endoprosthesis had to be removed due to recurrent PJI. In 14 patients no further surgical treatment was necessary. In Table 3 the organisms of 95 patients during the first stage revision with negative cultures during second stage are listed.
Table 4 shows the bacteria identified during first stage revision and during second stage revision and if another revision was necessary (n = 20).
Discussion
The data in the present study show that aspiration of synovial fluid with a PMMA spacer in place is not an appropriate method for excluding persistent infection. The sensitivity for detecting bacteria was only 5 %, white blood counts had a sensitivity of only 31.3 %. Furthermore CRP in serum sensitivity was also low with 42.1 % and the calculated cut off values were not very useful for clinical practice.
In two-stage revision surgery, the surgeon has to decide if reimplantation or another revision with debridement and spacer exchange has to be performed. While in some cases the decision for a spacer exchange instead of reimplantation is easy because of the clinical signs, in most cases no obvious clue is present. As shown in the literature and in our study measurements of CRP in serum and white blood counts in synovial fluid are not helpful in excluding a persistent infection [21–23]. One of the strongest criterions for PJI is the positive culture of tissue or joint fluid. We were using this criterion in accordance to the diagnosis of PJI when an antibiotic loaded spacer was in place [18, 24]. Recently it could be shown that the sensitivity of synovial cultures in Girdlstone hips was only 10 % [25].
Our results for cultures from synovial fluid were even worse (5 %).
The fact that bacteria are in a biofilm form and are adherent to foreign bodies is one reason for the uncertain predictability and maybe the local concentration of antibiotics is another reason, why even cultures of the joint fluid were not able to detect persistent infection. The period during which antibiotics are released is described as being up to several months. This has been demonstrated both in urine and locally [6, 8, 9].
It is therefore not possible to observe an antibiotic-free interval of at least 14 days for diagnosis prior to reimplantation.
In the diagnosis of a periprosthetic joint infection Fink et al. described a sensitivity of 100 % and a specificity of 98.1 % with a biopsy technique in knees [24]. There is no literature using biopsy techniques before reimplantation in knees and hips. But in two stage revision arthroplasty of the shoulder Zhang et al. found 22 % positive cultures before replantation [26].
This might be an approach to get more reliable results before the second stage with the disadvantage of an additional invasive procedure. Because of the results presented here we also investigated the Interleukin 6 (IL-6) in synovial fluid and in serum before reimplantation. In serum we found a useful cut-off-value to exclude persistent infection. IL-6 in serum ≤8 pg/ml had a negative predictive value of 92.1 %, a value of ≥13 pg/ml had a positive predictive value of 90.9 % [27].
Sonication of the implants seems to be a method with a higher sensitivity than conventional microbiological methods and identification of polymicrobial infections can be improved [28, 29]. Sonication of PMMA spacers also showed improvement of the sensitivity of intraoperative cultures from 45 to 82 % [30].
Unfortunately sonication cannot be used before second stage revision to decide if a spacer exchange or replantation is indicated.
Aggravating to the high number of persistent infections we found a high number of changing organisms. These data are supported by the findings of Cabo et al., who also found a large number of different and more resistant organisms during replantation [31]. The heterogeneous finding of pathogens during second stage reimplantation makes it difficult to find an explanation. Of course contamination can be a problem, but it would be very unusual in such a high percentage. Another point might be the change of patterns of resistance because of the long release of local antibiotics. Here we need further research about antibiotic concentrations locally and the risk of creating new resistances.
There are some limitations of the study. We did not look at comorbidities and risk factors for reinfection. We did not take into account if the patients had a PJI in history. The design of the study is retrospectively.
In conclusion, CRP in serum, white blood count in synovial fluid and even cultures from synovial fluid before replantation with a PMMA spacer in place seem to be uncertain parameters to exclude persistent PJI. We cannot recommend aspiration of synovial fluid before reimplantation anymore. Other biomarkers have to be investigated.
References
Swedish Hip Arthroplasty Register, Annual Report 2013
Herberts P (2000) Malchau, Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand 71(2):111–121
Meermans G, Haddad FS (2010) Is there a role for tissue biopsy in the diagnosis of periprosthetic infection? Clin Orthop Relat Res 468(5):1410–1417
McArthur BA, Abdel MP, Taunton MJ, Osmon DR, Hanssen AD (2015) Seronegative infections in hip and knee arthroplasty: periprosthetic infections with normal erythrocyte sedimentation rate and C-reactive protein level. Bone Joint J 97-B(7):939–944
Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L (2008) Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis 47(11):1403–1409
Frommelt L (2009) Diagnosis and treatment of foreign-body-associated infection in orthopaedic surgery. Orthopade 38(9):806–811
Anagnostakos K, Jung J, Schmid NV, Schmitt E, Kelm J (2009) Mechanical complications and reconstruction strategies at the site of hip spacer implantation. Int J Med Sci 6(5):274–279
Bertazzoni Minelli E, Benini A, Magnan B, Bartolozzi P (2004) Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother 53(2):329–334
Webb JC, Gbejuade H, Lovering A, Spencer R (2013) Characterisation of in vivo release of gentamicin from polymethyl methacrylate cement using a novel method. Int Orthop 37(10):2031–2036
Mortazavi SM, Vegari D, Ho A, Zmistowski B, Parvizi J (2011) Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Rel Res 469(11):3049–3054
Garvin KL, Evans BG, Salvati EA, Brause BD (1994) Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop Rel Res 298:97–105
Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP (2007) Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty 22(1):72–78
Romano CL, Gala L, Logoluso N, Romano D, Drago L (2012) Two-stage revision of septic knee prosthesis with articulating knee spacers yields better infection eradication rate than one-stage or two-stage revision with static spacers. Knee Surg Sports Traumatol Arthrosc 20(12):2445–2453
Pelt CE, Grijalva R, Anderson L, Anderson MB, Erickson J, Peters CL (2014) Two-stage revision TKA is associated with high complication and failure rates. Adv Orthop (Epub 2014 Dec 24)
Stammers J, Kahane S, Ranawat V, Miles J, Pollock R, Carrington RW, Briggs T, Skinner JA (2015) Outcomes of infected revision knee arthroplasty managed by two-stage revision in a tertiary referral centre. Knee 22(1):56–62
Ibrahim MS, Raja S, Khan MA, Haddad FS (2014) A multidisciplinary team approach to two-stage revision for the infected hip replacement: a minimum 5-year follow-up study. Bone Joint J 96-B(10):1312–1318
Kim YH, Park JW, Kim JS, Kim DJ (2015) The outcome of infected total knee arthroplasty: culture-positive versus culture-negative. Arch Orthop Trauma Surg 135(10):1459–1467
Parvizi J, Gehrke T, Chen AF (2013) Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 95-B(11):1450–1452
Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O (2002) The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg 91(2):178–181
Zimmerli W, Moser C (2012) Pathogenesis and treatment concepts of orthopaedic biofilm infections. EMS Immunol Med Microbiol 65(2):158–168
Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ (2011) What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Rel Res 469(4):1002–1008
Mont MA, Waldman BJ, Hungerford DS (2000) Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am 82-A(11):1552–1557
Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J (2009) Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Rel Res 467(7):1699–1705
Fink B, Makowiak C, Fuerst M, Berger I, Schäfer P, Frommelt L (2008) The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br 90(7):874–878
Janz V, Bartek B, Wassilew GI, Stuhlert M, Perka CF, Winkler T (2015) Validation of synovial aspiration in girdlestone hips for detection of infection persistence in patients undergoing 2-stage revision total hip arthroplasty. J Arthroplasty (Epub 2015 Sept 8)
Zhang AL, Feeley BT, Schwartz BS, Chung TT, Ma CB (2015) Management of deep postoperative shoulder infections: is there a role for open biopsy during staged treatment? J Shoulder Elbow Surg 24(1):e15–20
Hoell S, Borgers L, Gosheger G, Dieckmann R, Schulz D, Gerss J, Hardes J (2015) Interleukin-6 in two-stage revision arthroplasty: what is the threshold value to exclude persistent infection before re-implanatation? Bone Joint J 97-B(1):71–75
Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C (2013) Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop 37(5):931–936 (Epub 2013 Mar 24)
Janz V, Wassilew GI, Kribus M, Trampuz A, Perka C (2015) Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch Orthop Trauma Surg 135(10):1453–1457 (Epub 2015 Sep 8)
Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR (2014) Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res 472(7):2208–2214
Cabo J, Euba G, Saborida A, Gonzalez-Panisello M, Dominguez MA, Agullo JL et al (2011) Clinical outcome and microbiological findings using antibiotic-loaded spacers in two stage revision of periprosthetic joint infections. J Infect 63(1):23–31
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoell, S., Moeller, A., Gosheger, G. et al. Two-stage revision arthroplasty for periprosthetic joint infections: What is the value of cultures and white cell count in synovial fluid and CRP in serum before second stage reimplantation?. Arch Orthop Trauma Surg 136, 447–452 (2016). https://doi.org/10.1007/s00402-015-2404-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2404-6