Abstract
“Advanced core decompression” (ACD) is a treatment option for osteonecrosis of the femoral head (ONFH) that aims at complete removal of the necrotic tissue using a percutaneous expandable reamer and refilling of the head with an osteoconductive bone-graft substitute. The objective of this study was to evaluate if the success of ACD depends on the amount of necrotic tissue remaining after the procedure and how efficiently the necrotic tissue can be removed with the current reamer. Three-dimensional models of proximal femora including ONFH were generated from the preoperative MRIs of 50 patients who underwent ACD. Best-case removal was calculated by geometrical analysis. In 28 of 50 cases, postoperative MRI was used to determine how much necrotic tissue had been removed. Prognostic values and correlations were evaluated in order to assess success or failure of the treatment. The amount of preoperative and remaining necrosis correlates significantly with treatment failure. The larger both volumes are, the more likely it is that treatment will fail. In patients with remaining necrosis of less than 1000 mm3, no treatment failure was observed. The amount of necrosis actually removed differed significantly from the amount calculated as the best possible result. Simulation of the removal procedure showed that complete removal is not possible. These results led to the conclusion that the success of ACD depends on the amount of necrotic tissue remaining in the femoral head after the procedure. Modifications to the instrument are necessary to increase the amount of necrotic tissue that can be removed.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An estimated 10,000–20,000 new cases of osteonecrosis of the femoral head (ONFH) occur every year in the United States alone [1, 6, 11, 14, 18]. If left untreated, the femoral head collapses in more than 90 % of patients [17]. As this condition mainly affects patients in their third to fifth decade of life, hip-preserving techniques should preferably be used for treatment. Joint arthroplasty has only a limited survival and is therefore unsuitable in such cases [17]. Furthermore, there is no final consensus in the literature about the use of short-stem hip arthroplasty in patients with progressive ONFH [9]. The most commonly chosen surgical treatment option is core decompression, which is performed by drilling into the necrotic lesion to release pressure in the affected tissue and encourage ingrowth of new blood vessels [3, 8, 10]. Different variations of the conventional core decompression technique have been described, e.g. multiple drilling or core decompression in combination with bone marrow mononuclear cells, nano-hydroxyapatite/polyamide 66 rod or vascularised fibular grafts [2, 7, 13, 24]. However, an increasing number of surgeons now attempt to remove as much as possible of the necrotic tissue, as this procedure yields much better results [16, 19]. Rosenwasser et al., for example, curetted the necrotic material out through a window (“trapdoor”) in the femoral head and refilled the defect with cancellous bone [19]. They achieved a high success rate of 86 %. Mont et al. used a similar technique, but replaced the diseased bone with a bone-graft substitute (a combination of demineralized bone matrix, processed allograft bone chips and thermoplastic carrier) and reported a success rate of 87 % [16]. In comparison to core decompression, these procedures are much more invasive. Therefore, surgical methods are needed that enable removal of as much of the necrotic tissue as possible in a minimally invasive way.
“Advanced core decompression” (ACD) is a new option for treatment of ONFH that may fulfil this requirement [12]. It is based on the conventional core decompression technique, but is performed using a new percutaneous expandable reamer (X-REAM®, Wright Medical Technology™, Inc. Arlington, TN, USA). This instrument is intended to achieve almost complete removal of the necrotic tissue through a drilling canal. The X-REAM® can be expanded in situ by turning the adjustable mechanism (Fig. 1). After removal of the necrotic tissue, the drill hole and the defect are refilled with an osteoconductive, injectable bone-graft substitute consisting of calcium sulphate (CaSO4)–calcium phosphate (CaPO4) (PRO-DENSE®, Wright Medical Technology™, Inc. Arlington, TN, USA) [5, 12].
The outcome of ACD is reported to be better for small necrotic defects than for larger defects [12]. In the first pilot study, it was surmised that the X-REAM® may not enable complete removal of the necrosis [12]. Furthermore, remaining necrotic tissue was observed in nearly all of the cases included in that study. The current study is the first comprehensive investigation of the X-REAM®. The aims were (1) to evaluate if the preoperative necrotic volume (PNV) and remaining necrotic volume (RNV) are prognostic parameters in terms of ACD treatment failure, (2) to evaluate by means of an in silico simulation the best possible removal of different types and sizes of necrotic bone defects with the X-REAM® and (3) to evaluate how well this best possible removal could be achieved in patients who have previously undergone surgery.
Materials and methods
The study was approved by the local Ethics Committee (reference number 10-4293). Fifty patients with a mean follow-up of 32.91 months after ACD were included. The mean age at the date of surgery was 45.47 years. According to the ARCO classification, 40 patients had stage 2 and ten patients had stage 3 lesion. All patients underwent ACD at the Department of Orthopaedics of the University of Duisburg-Essen. All procedures were performed by one surgeon according to the surgical technique described previously [12]. To avoid the influence of the learning curve, the surgeon had already performed ten ACDs prior to the study. Furthermore, he had performed more than 30 conventional Core Decompressions before changing to the ACD procedure. A preoperative MRI was performed for all patients and no collapse of the femoral head was visible at the time of surgery. After ACD, all patients were advised to have a routine MRI scan about three months after surgery. Twenty-eight of 50 patients complied with this recommendation and provided their postoperative MRI for evaluation. This sub-group of patients had a mean follow-up of 38.53 months and a mean age of 50.17 at the date of surgery. Every patient underwent MRI shortly before and within 3 months after surgery.
The 50 preoperative MRIs were carried out using a 1.5Teslan MRI (Intera®, Philips Healthcare, Hamburg, Germany) according to a comprehensive protocol, developed for evaluation of ONFH, including coronal t1-weighted TSE (turbo spin echo) sequence, coronal t2-weighted TSE sequence, transverse proton density spectral attenuated inversion recovery (SPAIR), transverse short-tau inversion recovery (STIR) and coronal t1-weighted gradient echo (GRE) sequence after injection of gadolinium (Gd). The slice thickness was 3 mm except for t1 after Gd (2 mm). The postoperative MRIs were performed on the same 1.5Tesla MRI (Intera®, Philips Healthcare, Hamburg, Germany) in ten cases and in 12 cases on a 3Tesla-MRI (Skyra®, Siemens Healthcare, Erlangen, Germany) according to a comparable protocol with additional coronal 3D isotropic DESS sequence and sagittal PD/T2w TSE sequence. Six patients provided MRI images that were performed on external MRIs but according to comparable protocols.
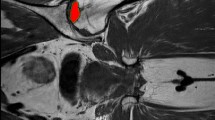
For simulation of the removal of the necrotic tissue and evaluation of different parameters, three-dimensional computer-aided design (CAD) models of proximal femora were generated from the preoperative MRI scan data using sophisticated segmentation and shape reconstruction techniques (Avizo®, Visualization Science Group, Burlington, MA, USA). The procedure included first a segmentation process that assigns the bone region to pixels of the image. A polygonal surface model was then extracted from segmentation results based on surface reconstruction techniques. The last step was the generation of the model volume enclosed by the surface. The preoperative necrotic volume (PNV) in the femoral head was generated from the same procedure for each femur model (Fig. 2). The extent of the necrosis on the preoperative MRI images was determined in a consensus read-out by two orthopaedic surgeons and an engineer specially trained for this project. All the models were then imported into the multi-platform CAD software Catia® V5 (Dassault Systèmes S.A., France) for geometric analysis. The entrance point of the drill hole was supposed at the inferior border of the greater trochanter in the subtrochanteric lateral cortex as the position of the supposed standard entrance point [23], and a line connecting this point and the centroid of the necrosis volume was created. A drill hole of 9 mm diameter was created based on the above line penetrating to the necrotic domain in the femoral head.
The working domain of the X-REAM® was defined as a spheroid with a fixed longitudinal extent of 15.9 mm and a flexible diameter of 9–21 mm (Fig. 3). This is the maximum working domain that the X-REAM® can achieve. The best possible removal (BPR) of necrosis was defined as the volume of the intersection part between the necrosis and the working domain of the reamer (Fig. 4, left). The necrotic tissue volume that remains after removal was defined as the minimum remaining necrotic volume (MRNV, Fig. 4, right). In order to get the BPR of the necrotic tissue, two conditions were supposed during the simulation. First, the centroid of the reamer working domain was fixed with that of the necrosis volume. Second, the entrance point of the drill hole was adjusted so that the removable volume of necrosis is maximized without increasing the risk of femur fracture. As recommended, the entrance point was located around its standard position with a maximum deviation to the distal direction of 20 mm [4, 20–23].
The actual remaining necrotic volume after ACD (RNV) was evaluated in the same way as for the preoperative necrotic volume for 28 postoperative MRIs of the 28 studied femora. The original necrotic area was transferred from the pre- to the postoperative MRIs to determine the extent of the remaining necrotic tissue. The difference between the preoperative and the actual remaining necrotic volume revealed the achieved removal of necrosis (AR). All volumes were recorded in [mm3]. To determine the proportion of the removed necrosis as a percentage, the ratio between BPR and AR was calculated. The need for a hip replacement during postoperative follow-up due to collapse of the femoral head or persisting respectively increasing pain was defined as treatment failure.
The percentage of treatment failure was determined not only for all 50 patients, but also for the sub-groups. Sub-groups were created according to PNV (limits 2500 and 5000 mm3) and according to RNV (limits 1000 and 2000 mm3).
Statistical analysis
Summary statistics of the data was expressed as mean ± standard deviation (SD). The Shapiro–Wilk Test was used to test for normal distribution of the data (p ≤ 0.05 ≥ not normal distribution). Depending on the distribution, the paired t test or Wilcoxon signed-rank test were used for evaluation of differences between the PNV and RNV as well as between the MRNV and RNV.
In addition, Kendall’s-tau-b correlation was performed with the independent variables PNV and RNV and treatment failure, which can be considered as a dependent variable. The prognostic values of PNV and RNV for treatment failure were studied using a receiver operating characteristic (ROC) curve. Comparisons with p values <0.05 were considered significant.
The software SPSS 19 (IBM, Ehningen, Germany) was used to carry out the statistical computations.
Results
The mean preoperative necrotic volume (PNV) of all patients (N = 50) was 4054 mm3 (range from 249 to 14,261 mm3; SD 3155 mm3). After ACD, the PNV of the 28 patients with an additional postoperative MRI had significantly (p < 0.001) decreased from 4220 mm3 to a mean actual remaining necrotic volume (RNV) of 2822 mm3. Simulations revealed that in these 28 patients, a mean minimum remaining necrotic volume (MRNV) of 2321 mm3 could have been achieved, which would have been significantly smaller than the actual RNV. Simulations of all 50 patients revealed a MRNV of 2184 mm3. Complete removal was not achieved in any case. The mean achieved removal (AR) of the twenty-eight patients with pre- and postoperative MRIs was 71.87 % (14–99; SD 19) of the best possible removal (BPR).
Kendall’s-tau-b correlation revealed that there is a significantly positive correlation between both the PNV and the RNV and treatment failure. In other words, the larger the PNV and RNV are, the more likely it is that the treatment will fail. The ROC curve was used to quantify limits for RNV and PNV that allow differentiation of the patients in whom the success of the treatment is doubtful. A PNV of 3266 mm3 and RNV of 2294 mm3 were determined as cut-off values for treatment failure, giving sensitivities of 72.7 and 75.0 %, and specificities of 73.1 and 73.3 %, respectively. In this context, the sensitivity describes the percentage of the patients correctly detected as positive for treatment failure after ACD and the specificity describes the percentage of patients correctly detected as negative for treatment failure.
The evaluation of the failure rate according to RNV revealed no treatment failure for patients with an RNV of less than 1000 mm3, but a percentage of 42.9 % treatment failure for RNV between 1000 and 2000 mm3 and 69.2 % for RNV larger than 2000 mm3.
For PNV, a failure rate of 13 % was observed with a PNV of less than 2500 mm3, 55 % with a PNV between 2500 and 5000 mm3 and 69.2 % with a PNV greater than 5000 mm3. Documentation of a typical case with pre-/postoperative X-rays and MRIs is given in Fig. 5.
a Preoperative X-ray and b MRI images of a study patient with a large defect (14,261 mm3) on the right side and a relatively small defect on the left side (1139 mm3). c After advanced core decompression, MRI shows a remaining necrotic volume of 10,864 mm3 on the right side and 140 mm3 on the left side. d In the further follow-up, the patient suffered from a collapse of the femoral head on the right side, whereas the left side was pain-free
Apart from treatment failures due to collapse of the femoral head or persisting, respectively, increasing pain, no complications have been observed. This also applies for potential accidental penetration of the femoral head.
Discussion
The positive correlations between the preoperative and remaining necrotic volumes (PNV and RNV) and treatment failure indicate that both the RNV and PNV are important prognostic parameters for the success of ACD. The PNV could be applied prior to ACD to predict if this is a meaningful procedure for the patient in question. The sub-division of groups according to size revealed that patients with a PNV of less than 2500 mm3 have a relatively low failure rate of 13 % in comparison to patients with a larger PNV. The ROC curve showed a quite high cut-off value for a PNV of about 3266 mm3, which means that this volume reflects the best ratio of the patients correctly detected as positive for treatment failure (sensitivity of 72.7 %) to the patients correctly detected as negative for treatment failure (specificity of 73.1 %).
However, RNV is the more important indicator as it can be influenced by ACD. For RNV, the ROC curve revealed 2294 mm3 as a cut-off value with an optimal ratio of sensitivity to specificity. For clinical practice, it is of even greater interest that the treatment did not fail in any of the patients with an RNV of less than 1000 mm3, whereas patients with RNVs between 1000 and 2000 mm3 and greater than 2000 mm3 had failure rates of 42.9 and 69.2 %, respectively. Therefore, the goal of ACD must be to achieve an RNV of less than 1000 mm3 after surgery. This is important, as PRO-DENSE®, the synthetic injectable bone graft used for this method, is only osteoconductive. This material therefore works only as a scaffold that needs the support of healthy bone in the vicinity to achieve successful bone remodelling.
The study also showed that ACD needs further modifications if it is to achieve the goal of an RNV of less than 1000 mm3. Firstly, drilling must be made more accurate, so that the reamer can target the lesion more precisely, thus enabling removal of larger amounts of necrotic tissue. The amount of necrotic tissue actually removed was a mean percentage of only 71.87 % of the best possible removal (BPR). This significant difference between the achieved removal (AR) and the BPR is remarkable, considering that preoperative planning was carried out for all patients and surgery was performed under fluoroscopic guidance by an experienced surgeon. Therefore, preoperative planning must be implemented better during surgery to improve intraoperative evaluation of the drilling and tissue removal. The main challenge is that necrotic lesions can best be seen in MRI, whereas preoperative planning and intraoperative guidance are performed using X-rays. An option for improvement may be the use of a navigation system, which enables preoperative planning and intraoperative navigation with a MRI dataset.
Secondly, the shape of the reamer needs to be re-designed. Our simulation of the best possible removal showed that the current shape of the X-REAM® would miss the target of complete removal in every case. Even if the positive correlation between the defect size and the minimum remaining necrotic volume (MRNV) suggests that the instrument just needs to be larger, an alteration to the size would not be sufficient. The main problem is that in order to remove the necrotic tissue, it is necessary to turn the X-REAM® around 360°. But a 360° turn can cause the reamer to make contact with cortical bone on one side although the necrotic tissue to be removed is on the other side. This means that the reamer has to be stopped before complete removal can be achieved to avoid damaging the cortical bone.
The study has some limitations. First, patients with stage 2 and 3 lesions according to the ARCO classification have been included in the study. This may affect the results, as it is known that stage 3 lesions have a worse outcome in comparison to stage 2 [15]. A second limitation is that as complete removal was not achieved, it was not possible to evaluate the effect of complete removal. Finally, the study lacks a control group treated by conventional core decompression.
Conclusion
The success of ACD depends on the RNV. To achieve the goal of almost complete removal of the necrotic tissue with an RNV of less than 1000 mm3, further efforts must be made to improve the technique of tissue removal, preferably in a minimally invasive manner.
References
National Center for Health Statistics (1994) 1990 and 1991 National Hospital Discharge Survey. The American Academy of Orthopaedics Surgeons Department of Research and Scientific Affairs, Rosemont, Illinois
Al Omran A (2013) Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg 133(5):609–613
Arlet J, Ficat P (1964) Forage-biopsie de la tete fémorale dans l’ostéonécrose primitive. Observations histo-pathologiques portant sur huit forages. Rev rhumat 31:257–264
Camp JF, Colwell CW Jr (1986) Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am 68(9):1313–1319
Civinini R, De Biase P, Carulli C et al (2012) The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head. Int Orthop 36(8):1583–1588
Coventry MB, Beckenbaugh RD, Nolan DR, Ilstrup DM (1974) 2,012 total hip arthroplasties. A study of postoperative course and early complications. J Bone Joint Surg Am 56(2):273–284
Fang T, Zhang EW, Sailes FC, McGuire RA, Lineaweaver WC, Zhang F (2013) Vascularized fibular grafts in patients with avascular necrosis of femoral head: a systematic review and meta-analysis. Arch Orthop Trauma Surg 133(1):1–10
Ficat RP (1985) Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 67(1):3–9
Floerkemeier T, Budde S, Gronewold J et al (2015) Short-stem hip arthroplasty in osteonecrosis of the femoral head. Arch Orthop Trauma Surg 135(5):715–722
Hungerford DS (1979) Bone marrow pressure, venography, and coredecompression in ischemic necrosis of the femoral head. In: The Hip proceedings of the sevens open scientific meeting of the hip society, St. Louis, C.V. Mosby, pp 218–237
Jacobs B (1978) Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthopa Relat Res 130:51–67
Landgraeber S, Theysohn JM, Classen T et al (2013) Advanced core decompression, a new treatment option of avascular necrosis of the femoral head–a first follow-up. J Tissue Eng Regen Med 7(11):893–900
Liu Y, Liu S, Su X (2013) Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg 133(1):125–133
Mankin HJ (1992) Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med 326(22):1473–1479
Mont MA, Carbone JJ, Fairbank AC (1996) Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res 324:169–178
Mont MA, Etienne G, Ragland PS (2003) Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res 417:84–92
Mont MA, Hungerford DS (1995) Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 77(3):459–474
Robinson HR, Jr., Springer JA (1992–1993) Success of core decompression in the management of early stages of avascular necrosis: a four-year prospective study. Orthop Trans 16:707
Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB (1994) Long term followup of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthopa Relat Res 306:17–27
Schneider W, Breitenseher M, Engel A, Knahr K, Plenk H Jr, Hofmann S (2000) The value of core decompression in treatment of femur head necrosis. Der Orthop 29(5):420–429
Smith SW, Fehring TK, Griffin WL, Beaver WB (1995) Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am 77(5):674–680
Steinberg ME (1995) Core decompression of the femoral head for avascular necrosis: indications and results. Can J Surg J canadien de chirurgie 38 (Suppl 1):S18–S24
Tran TN, Warwas S, Haversath M et al (2014) Experimental and computational studies on the femoral fracture risk for advanced core decompression. Clin Biomech (Bristol, Avon) 29(4):412–417
Yang P, Bian C, Huang X, Shi A, Wang C, Wang K (2014) Core decompression in combination with nano-hydroxyapatite/polyamide 66 rod for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg 134(1):103–112
Acknowledgments
The study was partly supported by a grant from the Bundesministerium für Wirtschaft und Technologie within the AiF program and partly by the German Research Foundation (DFG) (LA 2619/6-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
One of the authors is a consultant at Wright Medical Technology™, Inc. Arlington, TN, USA.
Rights and permissions
About this article
Cite this article
Landgraeber, S., Tran, T.N., Claßen, T. et al. Geometric analysis of an expandable reamer for treatment of avascular necrosis of the femoral head. Arch Orthop Trauma Surg 135, 1357–1362 (2015). https://doi.org/10.1007/s00402-015-2287-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2287-6