Abstract
Introduction
Venous thromboembolism (VTE) is a common complication after total hip arthroplasty (THA) or total knee arthroplasty (TKA) and may be the cause for a secondary PE and associated morbidity/mortality. We performed a systematic literature review of risk factors and risk reduction of VTE after THA or TKA.
Materials and methods
A systematic search of PubMed database, the Cochrane Library, OVID MEDLINE and American Academy of Orthopaedic Surgeons (AAOS), without restriction of publication data and language, was conducted. We performed a meta-analysis of ten factors for VTE after THA or TKA. Four authors independently assessed data extraction and quality of the studies using the Newcastle-Ottawa Scale (NOS) as quality assessment tool. Assessment of heterogeneity and analysis of data were operated by Review Manager 5.2.9.
Results
Fourteen retrospective case–control or prospective cohort studies, which included 18,075 patients who developed VTE after THA or TKA of a total of 1,723,350 cases, were selected. Our results demonstrated that, among all ten factors investigated, 3 main risk factors were significantly associated with VTE after THA or TKA: history of VTE (RR > 10.6), varicose vein (RR > 2.7) and congestive cardiac failure (RR 2). There was also an increase of VTE risk ranging from 8 to 30 % for female gender < age (≥80) < hypertension < (active) cancer < obesity (BMI ≥ 30) < (black) race. Data analysis revealed that diabetes mellitus had no significant relationship with VTE after THA or TKA.
Conclusions
This study highlighted the role of nine significant risk factors in the development of VTE after THA or TKA. Among all risk factors, history of VTE seems the one main indication for more potent anticoagulation. All other risk factors need to be considered and discussed with patients individually and balanced against the risk of bleeding and infection. Individual patient risk assessment, rather than a “blanket policy”, is considered the best management strategy before deciding on the type of chemical prophylaxis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE), which includes asymptomatic and symptomatic deep-vein thrombosis (DVT) and/or pulmonary thromboembolism (PE), has to be considered as a potentially serious complication after total hip arthroplasty (THA) and total knee arthroplasty (TKA). The vast majority of VTEs are asymptomatic, but can also be associated with long-term morbidity when compared with symptomatic events [1]. Patients with symptomatic VTE have a higher likelihood of prolonged hospital stay and a greater rate of re-hospitalization. Reduced quality of life and increased health care expenditures may also occur as a consequence [2].
Combined prevalence rates of 0.9–28 % for pulmonary embolism and 0.1–2 % for fatal pulmonary embolism following THA or TKA, respectively, have been reported [1]. Despite promotion and implementation of more potent and aggressive chemical prophylaxis, the rate of VTE-associated death has not been reduced over the last 30 years, especially after THA or TKA [1, 3]. Most guidelines [4–9] for prevention of VTE are presented in a general fashion including outcome of major orthopedic surgery with no detailed emphasis on TKA/THA surgery. Therefore, we targeted risk factors for patients undergoing after THA or TKA and more importantly wanted to assess the weighting of pre- and perioperative risk factors. Hence, we conducted a meta-analysis to assess the quality of available epidemiologic evidence, to evaluate the risk factors for VTE after THA or TKA in order to provide more information and improved guidance for surgeons and doctors managing this sub-group of patients and to allow for better risk stratification.
Methods
Search strategy and data source
A meta-analysis was conducted using PubMed database, the Cochrane Library, OVID MEDLINE and American Academy of Orthopaedic Surgeons (AAOS). The keywords were identified and MeSH descriptors or subject headings were arranged for the following search terms: “total hip arthroplasty”, “total hip replacement”, “THA”, “THR”, “total knee arthroplasty”, “total knee replacement”, “TKA”, “TKR”, “venous thrombus”, “venous thromboembolism”, “vein thrombosis”, “venous thrombosis”, “VTE”, “risk”, “risk assessment”. Articles which investigated the association between risk factors and VTE after THA or TKA were identified. An additional article was selected from references of relevant reviews and studies presented at major orthopedic journals such as the Journal of Bone and Joint Surgery (JBJS) and Japanese Orthopaedic Association (JOA). For further information, “Google Scholar” was also used. The evidence available from NICE [6], in particular the evidence tables [7] were also cross-checked and included for our analysis.
Study selection
According to the inclusion criteria presented in Table 1, all identified articles were reviewed independently on an initial screening of titles or abstracts to select potentially relevant articles. The results were cross-checked and then a second screening of all remaining full-text articles was reviewed by all of four authors separately to appraise the eligibility. The summarized results were cross-checked again. Any disagreements were resolved through discussion among all authors.
Quality assessment
Articles were reviewed by all of authors independently on separate occasions. The Newcastle-Ottawa Scale (NOS) was used as the quality assessment tool to evaluate the methodological quality of the studies included in the current review. Disagreements were resolved through discussion among all authors.
Data extraction
A specifically developed data-collection form was used to collect basic information of participants in each original study. The information from each study was recorded in the Table 2.
Odds ratios (OR) were regarded as the evaluation indicators of our meta-analysis. Extracted from original studies, the event and sample size were used to calculate the ORs value to devise forest plots. One author independently performed this procedure, resolving controversies by consensus with a second author.
Statistical analysis
Odds ratios (OR) and 95 % confidence interval (CI) were used as the common measures in our analysis. OR < 1 is negatively correlated with disease while it is positive when OR > 1. If OR equals 1, there is no statistical significance. Statistical significance was considered to be reached only if 95 % CI did not contain 1 or else no statistical significance existed. Calculated OR was used to make a list of weighting of risk factors.
The origins of heterogeneity consisted of differences in scores of quality, study design (prospective cohort or cases–control/retrospective), years of publication, characteristics of participants and prophylaxis across our studies. The presence of heterogeneity was assessed by Chi-squared test while the degree of heterogeneity was measured by I 2 statistic [10]. If P value calculated from Chi-squared test was less than 0.05, existence of statistical heterogeneity was affirmed. The statistical results with I 2 values provided an assessment level of heterogeneity. Less than 25 % was considered as little heterogeneity, 25–50 % as low level, 50–75 % as moderate level and exceeding 75 % as high level [11].
A fixed-effect model was employed for studies which showed homogeneity, while a random-effects model was used for those that did not. A sensitivity analysis was conducted to assess the stability of synthesis results and to identify sources of heterogeneity by removing every single study and analyzing the effect on overall results. Potential publication bias, which could affect the validity of the conclusion, was assessed by a funnel-plot. All analysis was performed by using Review Manager 5.2.9. P < 0.05 was considered statistically significant.
Results
Selection of studies
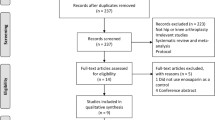
The initial search strategy yielded 1007 articles, 150 remained for screening based on the inclusion criteria after reviewing titles and abstracts. Of these 150 articles, 63 full-text articles were selected for further appraisal. After the assessment of full-text articles, 50 studies were excluded. Finally, 14 studies were included with one article retrieved from checking references lists. Table 2 provides a summary of characteristics of these studies, including author, year of publication, nation, study design, no. of patients, dropout rate, follow-up time, anesthesia, prophylaxis, operation side, diagnosis of VTE, and operation type (seen in Appendix Table 2). The entire selection process of articles according to PRISMA [12] Statement is presented in Fig. 1.
Study characteristics and results
Two studies [13, 14] designed as prospective cohort studies were included in this meta-analysis for data collection while other 12 included studies [2, 15–24] were designed as retrospective case–control studies. Ten articles [2, 14–18, 20, 23–25] related to participants undergoing THA or TKA and two articles [13, 22] only included THA patients, while the remaining articles [19, 21] only studied TKA patients. Among the 14 papers included in our article for data extraction and analysis about VTE, only in one article Ref. [25] made to the method of diagnosis of DVT or PE (either by venous Doppler ultrasonography or venography), but in the remainder [2, 13–24] no information was given as to how the diagnosis of VTE was confirmed. Meta-analysis was conducted for ten factors: gender, age, race, obesity, history of VTE, DM, malignancy, hypertension, CHF, varicose veins (seen in Appendix Table 3). Insufficient data were available for other risk factors (see NICE evidence tables) and hence no ORs could be calculated. The pooled ORs and 95 % confidence intervals of these risk factors were summarized in Table 4: five (age ≥ 70, race, DM, history of VTE, varicose veins) possessed significant heterogeneity, while the others showed no, low or moderate level of heterogeneity. Therefore, a random-effects model was preferred to pool the ORs for these five studies. As judged by Egger’s test, there was no evidence of publication bias being present at 5 % significance level for any of the risk factors.
Gender
Nine studies amounting to a total of 979,693 patients [2, 14–16, 21–25] provided eligible data. With statistics extracted from those 9 papers, the pooled OR calculated by the fixed-effect model was 0.93 (95 % CI 0.89–0.97, P = 0.0004 I 2 = 50 %) which revealed that females are more likely to have VTE after THA or TKA. Hence, our analyses revealed that females had a greater propensity to develop postoperative VTE after THA or TKA than males (shown in Fig. 2).
Age
Age as a risk factor was reported in eight inclusive studies [2, 13, 15, 16, 21, 23–25] with a total of 1,031,683 patients. Due to different categorization of age-groups in these studies, only 2 studies (a total of 120,989 patients) [15, 21] were included in the comparison of the age ≥ 60 and the age < 60, the fixed-effect pooled OR was 0.92 (95 % CI 0.80–1.05, P = 0.23) with no heterogeneity (I 2 = 0 %). To combine the effect sizes of these 8 studies, we compared the age ≥ 70 with the age < 70. The result showed a high level of heterogeneity (I 2 = 90 %) and the random-effect pooled OR was 0.97 (95 % CI 0.84–1.13, P = 0.73). However, significant difference was found between the age ≥ 80 and age < 80 (a total of 437,660 patients) [15, 16, 21, 25] in the incidence of VTE after THA or TKA. The result of the meta-analysis showed no heterogeneity (I 2 = 0 %) and the fixed-effect pooled OR for age ≥ 80 compared to age < 80 was 0.91(95 % CI 0.85–0.98, P = 0.01). In a conclusion, age ≥ 80 was a risk factor after THA or TKA while age ≥ 60 and age ≥ 70 did not increase the incidence of VTE (shown in Figs. 3, 4, 5).
Race
There were 3 studies (total of 922,051 patients) [14, 18, 23] providing extractable data and confirming that black race is a risk factor for VTE after THA or TKA. In assessment of this factor, the fixed-effect model was used and the pooled effect size (OR = 1.29, 95 % CI 1.19–1.39, P < 0.00001) demonstrated that VTE was related to the black race. As a result of a substantial heterogeneity (I 2 = 77 %), the random-effects model was used and pooled odd ratio for the black race was 1.32 (95 % CI 1.10–1.58, P = 0.002) in study effect size estimate (shown in Fig. 6).
Obesity
Two studies (total of 14,302 patients) [13, 17] with details of data referring to obesity were included. Significant difference in the incidence of VTE after THA or TKA was found between patients with BMI ≥ 30 and BMI < 30. It is found that BMI ≥ 30 was a risk factor to increase the morbidity of VTE. The result of the meta-analysis showed a low level of heterogeneity (I 2 = 27 %) and the fixed-effect pooled OR for BMI ≥ 30 compared to BMI < 30 was 0.78 (95 % CI 0.66–0.92, P = 0.003) (shown in Fig. 7).
History of VTE
Three studies (total of 153,196 patients) [13, 16, 21] provided available data to analyze the history of VTE. The fixed-effect pooled OR for patients with history of VTE compared to those without history of VTE was 11.87 (95 % CI 9.93–14.18, P < 0.00001), showing that patients with history of VTE were at a significant risk of VTE after THA or TKA. However, because of the high heterogeneity (I 2 = 98 %), the random-effects model was employed to estimate the pooled OR with an outcome of 10.67 (95 % CI 2.40–47.48, P = 0.002) (shown in Fig. 8).
Diabetes mellitus (DM)
Five studies (total of 598,216 patients) [16, 19, 21, 24, 25] were included. We used the fixed-effect model to evaluate the pooled effect size (OR = 0.94, 95 % CI 0.87–1.01, P = 0.09) which revealed no correlation between DM and postoperative VTE. However, there was a high-level heterogeneity (I 2 = 86 %) in these studies and thus the random-effects model was employed to estimate the pooled OR with the outcome of 1.02 (95 % CI 0.80–1.31, P = 0.88) (shown in Fig. 9).
Malignancy
Statistics from 5 studies (total of 465,758 patients) [2, 13, 21, 23, 24] referred to malignancy and significant difference was found from the pooled data between malignancy and postoperative VTE. The result showed that the fixed-effect pooled OR for the patients with “active” cancer compared to those without “active” cancer was 1.28 (95 % CI 1.01–1.62, P = 0.04) with a moderate-level heterogeneity (I 2 = 54 %) (shown in Fig. 10).
Hypertension
There were four studies (total of 539,965 patients) [14, 16, 23, 24] providing the extractable statistics of hypertension. In the fixed-effect model, hypertension was associated with an increased incidence of VTE (OR = 1.20, 95 % CI 1.07–1.34, P = 0.001). There was low level of statistical heterogeneity (I 2 = 35 %) (shown in Fig. 11).
Congestive heart failure (CHF)
Four studies (total of 522,449 patients) [13, 16, 24, 25] provided specific statistics. The result of our meta-analysis (OR = 2.03, 95 % CI 1.77–2.34, P < 0.00001) revealed that CHF was also significantly associated with a higher risk for postoperative VTE with a low level of heterogeneity (I 2 = 32 %) (shown in Fig. 12).
Varicose veins
Two studies (a total of 223,249 patients) [16, 20] provided specific statistics. We used the fixed-effect model to evaluate the pooled effect size (OR = 2.60, 95 % CI 1.74–3.88, P < 0.00001) which revealed patients with varicose veins were at an elevated risk of VTE. However, there was a large amount of heterogeneity (I 2 = 82 %) in these studies and thus the random-effects model was employed to estimate the pooled OR with the outcome of 2.76 (95 % CI 1.08–7.06, P = 0.03) (shown in Fig. 13).
Main findings
The results from our meta-analyses of 14 studies showed that mainly history of VTE (RR > 10) and varicose veins and CHF (RR > 2) have to be considered as the most significant risk factors for postoperative VTE after THA and THA. Gender (female), age (≥80), obesity, race (black), active malignancy, and hypertension, and were only associated with a ‘marginally’ increased VTE risk (RR 1.08–1.32). Age (≥60), age (≥70) and DM did not correlate with postoperative VTE risk.
Discussion
The results of this meta-analysis showed an increased risk for VTE after THA and TKA for the following factors: history of VTE > varicose veins > CHF > black race > BMI ≥ 30 > malignancy > hypertension > age over 80 > female gender. According to the Scottish national guideline of prevention and management of venous thromboembolism [4] and the NICE guidance [6, 7], most of these factors have also been previously identified to increase the risk/incidence of VTE. Our results do not support the SIGN guideline’s [4] suggestion that male gender, age (≥60; ≥70) are relevant risk factors for VTE. Furthermore, our analysis also suggests that the black race seems to be a risk factor which cannot be found in the guideline [4]. As for race, genetic factors may contribute to the finding of black race as an independent predictive factor for increased complications following THA and TKA. Some studies [26, 27] also revealed that the black race has higher incidence of VTE given that higher factor V Leiden III levels [e.g., (150 IU/dl)] are reported to occur more frequently among the black race than in Caucasians [27]. As a ‘new’ finding and in contrast to previous publications [4, 26], our analysis suggests that female, rather than male gender has a higher morbidity of VTE after THA or TKA. A study Silverstein et al. [28] showed the incidence of deep-vein thrombosis remained constant over time in male, while it decreased for females <55 years and increased for females >60 years [28]. Similarly, another study has reported that the incidence of VTE among men over age 80 was lower compared to older women [29]. In our meta-analysis, most of the patients undergoing THA or TKA were over age 60 (number of patients over 60: under 60 = 21:1), which may be the reason why our analysis suggested female being a risk factor of VTE after THA or TKA. In any case, the evaluation of male or female gender as a risk factor seems more closely associated with age rather than a gender-specific risk factor.
Advanced age is generally regarded as a risk factor for VTE [4]. In our analysis, the result did not show a higher incidence of VTE among patients at age over 60 or even over 70. However, patients >80 were at a higher risk of VTE after THA or TKA. Anderson et al. and Montagnana et al. [26, 30] also showed that the incidence of VTE increases with age. In keeping with our findings, Onur et al. and Baser et al. [2, 23] also demonstrated the obvious rise in the incidence of VTE among patients over 80. Thus more attention should be paid to VTE prophylaxis in patients of advanced age especially for those over 80.
Obesity (BMI ≥ 30) has been confirmed as a risk factor in our analysis which is in accordance with statements in the guidelines [4, 6]. Other studies also show obesity to be correlated with VTE risk, but only two articles were included in our meta-analysis to extract available statistics because others lacked unified group division of weight and there is different categorization of weight-groups. Some studies suggested that obesity may contribute to the risk of VTE by decreasing antithrombin III levels and fibrinolytic activity and is associated with venous stasis and with higher levels of prothrombotic factors such as fibrinogen, plasminogen activator inhibitor I and factor VII. In addition, obese patients have metabolic disturbance, are less active and more prone to being hospitalized for medical conditions or surgery, with the concomitant immobilization that may lead to VTE [31–33]. As a result, further studies may be needed to give a more persuasive statistical result. Some studies [34, 35] came to the same conclusion as our study that diabetes (DM) could not be considered as a risk factor. However, other studies revealed that DM is often associated with increased levels of procoagulant factors and the inhibition of endogenous fibrinolysis [36, 37] to increase incidence of VTE while Walter Ageno [38] found that DM was a risk factor of VTE by statistical analysis.
As is commonly acknowledged, malignancy was confirmed as risk factor for VTE after THA or TKA in our study. It has been suggested that the absolute risk is associated with the tumor type, the stage or extent of the cancer, and treatment with antineoplastic agents [39]. One of the hypotheses explained that “active” cancer cells can produce a cancer procoagulant and tissue factor (TF), which can induce a dose-dependent platelet activation by a mechanism similar to thrombin generation, and also release more cytokines and chemokines, such as vascular endothelial growth factor, interleukin-1 and tumor necrosis factor-alpha (TNF-alpha), which act on endothelial cells and leucocytes to synthesize and express TF and other cell adhesion molecules that predispose to VTE [13].
In our study, CHF (RR > 2) and to a lesser extent hypertension were risk factors for postoperative VTE which might be ascribed to blood hypercoagulable state [40–45]. CHF probably reflect both reduced patient mobility and impaired venous flow/return, hence similar to varicose veins.
Varicose veins (VVs) has been identified to increase the risk by >2.5 in our analysis, VVs can increase the backpressure in the venous system, resulting in valvular incompetency, both in the superficial veins and at the junctions between the deep and superficial systems [46]. Valvular incompetency can result in venous stasis and vessel damage within the superficial venous system and this is transmitted to the deep veins via the perforators [20]. We found that all related studies had not differentiated between superficial and deep (asymptomatic vs symptomatic) thrombosis. Therefore, the role and importance of VVs remains somewhat uncertain and further and better data are required to conclusively answer this question.
For rheumatoid arthritis (RA) recent literature suggests a protective effect of RA in comparison to OA and only an increased risk for patients, who are on anti-TNF DMARD medication [47]. The use of nonsteroidal anti-inflammatory drugs (NSAID) was considered as the main reason to explain this phenomenon, but this assumption is more than questionable given the findings by Niki et al. [48], who found that the incidence of DVT was equivalent in the two groups when the patients were matched for age and NSAID use. Importantly, however, Kawakami et al. [47] found that perioperative interruption of TNF blockers decreased the rate of SSI (surgical site infection) and DVT development in RA patients following major orthopedic surgery. Further well-designed studies with larger sample sizes [5] will be needed to evaluate these controversial risk factors.
As mentioned in NICE [6], prolonged immobilization was also identified as a risk factor of DVT or PE for decreasing the venous return and resulting in venous stasis [22]. It may well be that the majority of identified factors, e.g., age > 80, CHF and obesity are important indicators for poorer pre- and also postoperative mobility. Further studies with emphasis on mobility, e.g., using computer-based devices/pedometers, would shed more light into this incompletely answered topic.
Although risk assessment of VTE and relevant prophylactic guidance have been well presented in SIGN and NICE guidelines [4, 6], these still lack complete weighting of all risk factors. We have re-assessed the NICE evidence tables and have been unable in conjunction with our own data to produce valid Forest plots for some other risk factors, e.g., contraception, HRT and others poor mobility. Although THA or TKA as surgical procedure have been considered as risk factors for VTE per se, there is insufficient evidence to show whether the interactions between risk factors are additive or greater as has been previously suggested [4]. However, despite the lack of quality data and hence the absence of related forest plots/RRs, we do not suggest that the other suggested risk factors by SIGN/NICE do not exist or can be ignored.
Limitations
We have to recognize and point out some study limitations:
-
1.
Among 14 studies taken in, 9 provided follow-up time while the rest did not. Quality of included studies may be slightly affected by the incomplete data of follow-up time.
-
2.
The following reasons may contribute to heterogeneity in this paper. Firstly, individual differences exist in the study population of our analysis, for example, authors from 6 countries did the researches whose data comprised our analysis and racial difference may contribute to heterogeneity. Secondly, we noticed that heterogeneity was also owed to the variety of operative methods and used VTE prophylaxis (as showed in Table 2) following THA or TKA;
-
3.
It is acknowledged that the vast majority of VTEs are asymptomatic DVT and risk factors may have different, i.e., direct or indirect (or indeed a combination of both) influence on the incidence of symptomatic and asymptomatic DVT and PE. However, among the 14 crucial papers included in our article for data extraction and analysis about VTE, only one [17] can be used to differentiate between symptomatic and asymptomatic DVT and PE. Seven articles mentioned the difference between them but gave no data [13–16, 20–22] the remaining six [2, 18, 19, 23–25] did not even acknowledge this clinically relevant distinction. In order to improve the efficiency of prevention of VTE and avoiding death, more attention should be paid to symptomatic and asymptomatic DVT and PE after THA or TKA in future studies. More data will also be needed to differentiate between DVT and PE from total VTE patients (forest plots per risk factor see Fig. 14, RRs in Table 5) to enable better risk stratification and more selective chemical prophylaxis.
Table 5 Weighting of risk factors for VTE after THA or TKA (data include asymptomatic and symptomatic DVT-PE)
Conclusions
Despite these methodological limitations, given the large number of patient numbers included, we believe that our article contributes to the current understanding by providing weighting of risk factors for VTE after THA or TKA.
Importantly, however, our meta-analysis analysis suggests that mainly a history of VTE (RR > 10)—irrespective of family or individual history, varicose veins (RR 2.76) and CHF (RR 2.03) have to be considered as the most important risk factors and hence indication for potent chemical prophylaxis. All other factors identified may be considered as more relative indications.
We accept that our data did not include VTE prophylaxis and hence no conclusive recommendations can be made. However, when considering the most up-to date ACCP guideline recommendations from 2012 [9], which include Aspirin and when considering the evidence from NICE (evidence tables), that more potent anticoagulation only marginally reduced the VTE incidence (when compared with Aspirin plus mechanical prophylaxis), but substantially increases the risk of bleeding, it seems reasonable to conclude that routine prescription of potent chemical prophylaxis in the absence of the high-risk factors identified does not seem to be justified. Individual patient risk assessment, rather than a “blanket policy”, is considered the best management strategy before deciding on the type of chemical prophylaxis in addition to mechanical means to try and balance potential benefits and risks (bleeding and infection) associated with more potent chemical agents.
References
Bosque JJ, Coleman SI, Di Cesare P (2012) Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics 35(228):234
Baser O, Supina D, Sengupta N, Wang L, Kwong L (2010) Impact of postoperative venous thromboembolism on medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm 67:1438
Markovic-Denic L, Zivkovic K, Lesic A, Bumbasirevic V, Dubljanin-Raspopovic E, Bumbasirevic M (2012) Risk factors and distribution of symptomatic venous thromboembolism in total hip and knee replacements: prospective study. Int Orthop 36:1299
Healthcare Improvement Scotland (2010) Prevention and management of venous thromboembolism. http://www.sign.ac.uk/guidelines/fulltext/122/index.html
Samama MM, Dahl OE, Quinlan DJ, Mismetti P, Rosencher N (2003) Quantification of risk factors for venous thromboembolism: a preliminary study for the development of a risk assessment tool. Haematologica 88:1410
Hill J, Treasure T (2007) Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients having surgery: summary of NICE guidance. BMJ 334:1053
Venous Thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. http://www.ncbi.nlm.nih.gov/books/NBK116511/
MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, McLeod S, Bhatnagar N, Guyatt GH (2012) Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141:e1S
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ (2012) Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141:7S
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336
Beksac B, Gonzalez DVA, Salvati EA (2006) Thromboembolic disease after total hip arthroplasty: who is at risk? Clin Orthop Relat Res 453:211
Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D (2005) A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 129:87
Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP (2014) The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J 96-B:479
Wu PK, Chen CF, Chung LH, Liu CL, Chen WM (2014) Population-based epidemiology of postoperative venous thromboembolism in Taiwanese patients receiving hip or knee arthroplasty without pharmacological thromboprophylaxis. Thromb Res 133:719
Friedman RJ, Hess S, Berkowitz SD, Homering M (2013) Complication rates after hip or knee arthroplasty in morbidly obese patients. Clin Orthop Relat Res 471:3358
Adelani MA, Archer KR, Song Y, Holt GE (2013) Immediate complications following hip and knee arthroplasty: does race matter? J Arthroplasty 28:732
Adams AL, Paxton EW, Wang JQ, Johnson ES, Bayliss EA, Ferrara A, Nakasato C, Bini SA, Namba RS (2013) Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am 95:481
Dua A, Neiva S, Sutherland A (2012) Does previous varicose vein surgery alter deep vein thrombosis risk after lower limb arthroplasty? Orthop Surg 4:222
Pedersen AB, Mehnert F, Johnsen SP, Husted S, Sorensen HT (2011) Venous thromboembolism in patients having knee replacement and receiving thromboprophylaxis: a Danish population-based follow-up study. J Bone Joint Surg Am 93:1281
Kang BJ, Lee YK, Kim HJ, Ha YC, Koo KH (2011) Deep venous thrombosis and pulmonary embolism are uncommon in East Asian patients after total hip arthroplasty. Clin Orthop Relat Res 469:3423
Baser O, Supina D, Sengupta N, Wang L, Kwong L (2011) Clinical and cost outcomes of venous thromboembolism in Medicare patients undergoing total hip replacement or total knee replacement surgery. Curr Med Res Opin 27:423
Guijarro R, Montes J, San RC, Arcelus JI, Barillari G, Granero X, Monreal M (2011) Venous thromboembolism and bleeding after total knee and hip arthroplasty. Findings from the spanish national discharge database. Thromb Haemost 105:610
Kapoor A, Labonte AJ, Winter MR, Segal JB, Silliman RA, Katz JN, Losina E, Berlowitz D (2010) Risk of venous thromboembolism after total hip and knee replacement in older adults with comorbidity and co-occurring comorbidities in the Nationwide Inpatient Sample (2003-2006). BMC Geriatr 10:63
Montagnana M, Favaloro EJ, Franchini M, Guidi GC, Lippi G (2010) The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis 29:489
White RH, Keenan CR (2009) Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res 123(Suppl 4):S11
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LR (1998) Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 158:585
White RH, Zhou H, Murin S, Harvey D (2005) Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost 93:298
Anderson FJ, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation 107:I9
Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S (1996) Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 93:106
Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR (2002) Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 162:1182
Zhang ZJ, Zhao XY, Kang Y, Zhang ZQ, Yang ZB, He AS, Fu M, Sheng PY, Liao WM (2012) The influence of body mass index on life quality and clinical improvement after total hip arthroplasty. J Orthop Sci 17:219
Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LR (2009) Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arterioscler Thromb Vasc Biol 29:1399
Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, Henke PK (2007) Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg 45(335):341
Colwell JA, Nesto RW (2003) The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care 26:2181
Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL (2001) Platelet dysfunction in type 2 diabetes. Diabetes Care 24:1476
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW (2008) Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 117:93
Lee AY, Levine MN (2003) Venous thromboembolism and cancer: risks and outcomes. Circulation 107:I17
Jafri SM (1997) Hypercoagulability in heart failure. Semin Thromb Hemost 23:543
Jafri SM, Ozawa T, Mammen E, Levine TB, Johnson C, Goldstein S (1993) Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur Heart J 14:205
Sbarouni E, Bradshaw A, Andreotti F, Tuddenham E, Oakley CM, Cleland JG (1994) Relationship between hemostatic abnormalities and neuroendocrine activity in heart failure. Am Heart J 127:607
Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, Lipinska I, D’Agostino RB, Wilson PW (2000) Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA 283:221
Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA (2000) Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA 283:1145
Sheetz MJ, King GL (2002) Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288:2579
Thomson H (1979) The surgical anatomy of the superficial and perforating veins of the lower limb. Ann R Coll Surg Engl 61:198
Kawakami K, Ikari K, Kawamura K, Tsukahara S, Iwamoto T, Yano K, Sakuma Y, Tokita A, Momohara S (2010) Complications and features after joint surgery in rheumatoid arthritis patients treated with tumour necrosis factor-alpha blockers: perioperative interruption of tumour necrosis factor-alpha blockers decreases complications? Rheumatology (Oxford) 49(2):341–347
Niki Y, Matsumoto H, Hakozaki A, Mochizuki T, Momohara S (2010) Rheumatoid arthritis: a risk factor for deep venous thrombosis after total knee arthroplasty? Comparative study with osteoarthritis. J Orthop Sci 15:57
Acknowledgments
Thanks to Professor Jing Tian for his enlightenment in the process of selection of the topic and for his support to revise our paper as well as to give us valuable comments on the draft version of the paper despite his busy schedule. His instruction gave us great help in the completion of our study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
All the five authors contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Zhang, J., Chen, Z., Zheng, J. et al. Risk factors for venous thromboembolism after total hip and total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg 135, 759–772 (2015). https://doi.org/10.1007/s00402-015-2208-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2208-8