Abstract
Introduction
Femoroacetabular impingement (FAI) is a recognised cause of secondary osteoarthritis of the hip. Several imaging methods have been used to analyse the pathologic signs. Because of the lack of precise pre- and intraoperative overview and the difficulty locating osseous pathologies, arthroscopic and minimal invasive treatment is still challenging, even for trained surgeons. This paper describes a procedure that is based on magnetic resonance arthrography (MRA) and is used to virtually verify the range of motion (ROM) of the hip. It enables the evaluation of FAI and the preoperative simulation of adequate surgical manoeuvres.
Methods
Each MRI was completed on a 3.0 T system using a flexible transmit/receive surface body coil with the patient in the supine position. An axial three-dimensional (3D) gradient-echo (VIBE, volume interpolated breathhold examination) sequence was performed. For the generation of 3D bone models, semiautomatic segmentation of the MRA data was accomplished using Amira® visualisation software version 5.2. The self-developed software “HipProject”, written in C++, computes the maximal ROM of the hip. The virtual colliding regions were visualised for verification and simulation of osseous trimming.
Results
In addition, for necessary information about damage to the cartilage and labrum, “black bone” MRA was used to generate extremely precise 3D reconstructions of the hip joint to automatically calculate the preoperative osseous ROM. Furthermore, the acetabular and femoral locations of the impingement zone were individually visualised and quantified.
Conclusions
The described procedure is a useful tool for the preoperative investigation of impinging hips. It enables appropriate planning of required surgical interventions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Femoroacetabular impingement (FAI) is a recognised cause of intra-articular pathology and secondary osteoarthritis of the hip in young people [8, 13]. Three types of FAI have been described. “Cam-Impingement” has a predilection for younger adult males, especially those who are physically active [2, 15]. It is created by the collision between a prominent portion of a nonspherical femoral head and the acetabulum resulting in a selective delamination and failure of the articular surface of the acetabulum with relative preservation of the antero-superior labrum [2, 8, 15]. Pincer-impingement is more common in middle-aged women, occurring at an average age of 40 years. It develops in the setting of direct linear contact with an abrupt stop between the femoral head–neck junction and a localised anterior osseous acetabular prominence (acetabular retroversion) or a generally overcovered acetabulum (protrusio acetabuli) [13]. Most patients (86 %) develop a combination of both forms, which is called “mixed pincer and cam impingement”. A minority (14 %) of patients exhibit the pure femoroacetabular impingement forms of either cam or pincer impingement [15].
Several imaging methods have been suggested for analysing the pathologic signs of cartilaginous and labral damage. Standard conventional radiographic imaging is used to evaluate the bony hip morphology for abnormalities associated with impingement and to exclude arthritis, avascular necrosis and other joint problems. Magnetic resonance arthrography (MRA) is generally required to identify damage to the cartilage and labrum and to measure the alpha angle [1, 4, 7].
Furthermore, three-dimensional (3D) reconstructions of the bony surfaces based on computed tomography (CT) provides a survey of the hip to visualise the bony relationship between the femoral head and the acetabulum. However, because of the lack of precise pre- and intraoperative overview and the difficulty of locating osseous pathologies, the arthroscopic and minimal invasive treatment is still challenging, even for trained surgeons [2, 6]. Moreover, objective statements about the extent of bony debridement and the resulting ROM deficits cannot be verified using conventional imaging methods. Therefore, in 2007, Tannast et al. [14] described a ‘‘dynamic’’ imaging method for simulating hip ROM and individual femoroacetabular impingement location. Based on CT scanning of the pelvis and the femoral condyles, Tannast et al. used reconstructed 3D models of the pelvis and the femur to automatically calculate the preoperative osseous ROM. Furthermore, the acetabular and femoral locations of the impingement zone could be visualised and quantified. Although the gold standard for the generation of precise 3D bone models is CT [10], the high radiation dose is a main problem and should be considered, especially in young patients. For this reason, CT plays a less prominent role in current diagnostic approach to FAI. To address this problem, this paper describes a procedure that uses MRA data to verify the hip ROM for evaluation of FAI and simulation of adequate surgical manoeuvres implemented in the current routine diagnosis and treatment of FAI.
Methods
After a sterile injection of 15 ml of MRI contrast agent into the left hip-joint, the MRA investigation is performed on a 3.0 T system (Skyra; Siemens, Erlangen, Germany). Imaging is performed using a flexible transmit/receive surface body coil with the patient in the supine position. Following our standard MRI protocol, we perform an axial 3D gradient-echo (VIBE, volume interpolated breathhold examination) sequence (TR 10 ms, TE 4.92 ms, flip angle 10°, FOV 30 cm, matrix 512 × 486) with a spatial in plane resolution of 0.59 × 0.59 and a slice thickness of 0.8 mm. During the investigation, the leg is placed in a neutral position.
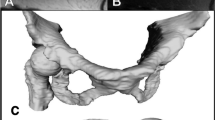
Standardisation is performed using Amira® visualisation software version 5.2 (Visage Imaging GmbH, Berlin, Germany), with semiautomatic segmentation of the loaded MRA data set. In each individual MRA slice, the bony structures are manually marked and stored. Therefore, a grey-scale based threshold function permits automatic recognition of bone borders and thus facilitates the segmentation process as previously described [9]. The prerequisite for this feature is that the data must be cropped to a sufficient joint excerpt to minimise the data volume to accelerate the segmentation process (Fig. 1). However, indistinct regions must later be corrected manually. Based on the segmentation data, the software then generates a precise 3D reconstruction of the hip joint in standard triangulated language format (STL).
The “HipProject” software is entirely written in the C++ programming language. Its architecture is based on free available software libraries such as Qt, VCollide and VTK. For the computation of the range of motion, we approximate the femoral head as a sphere that rotates around its centre at three immutable axes. Therefore, points on the femoral head of the bone model must be manually marked. The program computes the sphere on whose surface the points are closest. The centre of the sphere represents the rotation centre. The next step is to determine the axes for abduction and adduction, internal and external rotation as well as flexion and extension. Therefore, the pelvic anterior plane as a reference must be defined by marking the left and right spina iliaca anterior and tuberculum pubicum.
Then, the maximal range of motion can be computed. This is defined as the maximal rotation around a specific axis without colliding particles. These particles are the triangles of the 3D models as a result of the standard triangulated language format. Small irregularities in the model can be corrected by defining a number of ignorable collisions so that a small colliding part is ignored. The colliding regions can be visualised for verification and simulation of osseous trimming. It is possible to mill off the 3D model and determine how strong the changes in the range of motion will be. In this procedure, the marked colliding region at the bone can be manually erased. Then, an instant recomputation of the collision is performed for each alteration.
Results
For this paper, we investigated a left hip joint of a 30-year-old male patient with restricted internal rotation and reproducible pain occurring during forced internal rotation in 90° of flexion. The MRA shows a flattening of the anterior femoral head–neck junction with an alpha angle of 77° that was consistent with the clinically diagnosed CAM-impingement. There were findings of small cystic changes at the anterolateral femoral neck and a small cleft in the labral–chondral transitional zone. Other than multiple acetabular cysts, no chondral lesion of the femoral head articular cartilage was found (Fig. 2). In addition to the information about the cartilage and labrum of the hip joint, the MRA could be used to generate a 3D bone model as the result of the semiautomatic segmentation process. The software “HipProject” calculated the following ROM values: flexion/extension, 105°/50°; internal/external rotation in 90° of flexion, 11°/91°; and abduction/adduction, 70°/41°. The main typical colliding femoral region was visualised at 11° of internal rotation in 90° of flexion, which is consistent with the aforementioned clinical signs (Fig. 3). The virtual trimming of the femoral impingement zone (Fig. 4) leads to an increased internal rotation in 90° flexion only up to 20° (+9°). This is due to a second colliding acetabular region (Fig. 5). The virtual trimming of both zones causes an improved flexion of 113° and a greater internal rotation of 28° in 90° of flexion.
Discussion
Femoroacetabular impingement (FAI) is a recognised cause of secondary osteoarthritis of the hip. Although several imaging methods exist to visualise the pathologic signs, because of the lack of precise pre- and intraoperative overview and the difficulty locating osseous pathologies, the arthroscopic and minimally invasive treatment is still challenging. Incomplete or defective surgical trimmings cause several clinical and surgical problems connected with a high rate of revision hip arthroscopy [5, 6, 8]. Furthermore, femoral neck fractures may occur after over-resection of the femoral neck for treatment of CAM deformities [6]. Therefore, it is necessary to improve the diagnostic procedure to understand preoperatively the 3D dynamic problems of FAI.
The present study describes an MRA-based method that can be implemented in the current routine diagnosis of FAI. The self-developed software “HipProject” calculates the ROM and allows the visualisation of impingement zones and preoperative simulation of adequate surgical manoeuvres. The basic requirement is a precise 3D model of the hip joint using MRA data in a specific “black bone” sequence with high contrast and resolution levels. It is well known that CT data are the gold standard for the generation of precise 3D bone models. Nevertheless, Rathnayaka et al. [10] identified no significant difference in the accuracy of the results generated using semiautomatic grey-scale-based processes for MR versus CT data of long bones. However, MRA data do require manual correction, particularly in joint regions with areas of diffuse borders of various materials.
Several reports have described computer-assisted measurements of hip ROM with simulated hip joint motion [3, 11, 12, 14]. None of these applications allows a preoperative virtual simulation of the surgical trimming of osseous impingement zones to ultimately produce an effect on ROM. From our point of view, virtual surgical manoeuvres offer highly valuable information that can specifically inform the surgical planning and its practical implementation. The example of our CAM-hip has shown that the ROM was still not sufficiently improved by a single trimming of the femoral neck. Only when the acetabular osteophyte was taken into account does the simulated surgical manoeuvre provide a satisfactory increase in the ROM. Prospectively, the application can be incorporated into surgical navigation systems to improve arthroscopic performance.
One major limitation of our study is the fact that the method considers only the osseous restricted ROM, ignoring cartilaginous structures and soft-tissue. Furthermore, hips with nonconcentric joint morphology, such as dysplastic hips or advanced coxarthrosis, do not only have a pure rotation but also an additional translation. For these cases, the “HipProject” software is not usable whereby advanced osteoarthritis represents a contraindication for the surgical correction of FAI.
Conclusion
The described procedure is a useful tool for the preoperative investigation of impinging hips. It enables appropriate planning of the required surgical intervention and can be implemented in the current routine diagnosis of FAI using MRA. The application software system consists of the commercial visualisation software Amira® version 5.2 and the self-developed program code based on free available software libraries. In the future, the transmission of the virtually computed data into a surgical navigation system is planned to improve the arthroscopic performance.
References
Anderson LA, Peters CL, Park BB, Stoddard GJ, Erickson JA, Crim JR (2009) Acetabular cartilage delamination in femoroacetabular impingement. Risk factors and magnetic resonance imaging diagnosis. J Bone Joint Surg Am 91(2):305–313
Byrd JW, Jones KS (2009) Arthroscopic femoroplasty in the management of cam-type femoroacetabular impingement. Clin Orthop Relat Res 467(3):739–746
Charbonnier C, Chague S, Ponzoni M, Bernardoni M, Hoffmeyer P, Christofilopoulos P (2014) Sexual activity after total hip arthroplasty: a motion capture study. J Arthrop 29(3):640–647
Groh MM, Herrera J (2009) A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med 2(2):105–117
Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT (2007) Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy 23(12):1295–1302
Ilizaliturri VM Jr (2009) Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res 467:760–768
Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J (2002) The contour of the femoral head–neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br 84(4):556–560
Nouh MR, Schweitzer ME, Rybak L, Cohen J (2008) Femoroacetabular impingement: can the alpha angle be estimated? AJR Am J Roentgenol 190(5):1260–1262
Radetzki F, Mendel T, Noser H, Stoevesandt D, Rollinghoff M, Gutteck N et al (2013) Potentialities and limitations of a database constructing three-dimensional virtual bone models. Surg Radiol Anat 35(10):963–968
Rathnayaka K, Momot KI, Noser H, Volp A, Schuetz MA, Sahama T et al (2012) Quantification of the accuracy of MRI generated 3D models of long bones compared to CT generated 3D models. Med Eng Phys 34(3):357–363
Richolt JA, Teschner M, Everett PC, Millis MB, Kikinis R (1999) Impingement simulation of the hip in SCFE using 3D models. Comput Aided Surg 4(3):144–151
Sugano N, Yamanashi W, Sasama T (2003) Ranges of motion in anatomically normal hips using computer collision detection. 49th Annual Meeting of the Orthopaedic Research Society, New Orleans
Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA (2008) Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res 466(2):273–280
Tannast M, Kubiak-Langer M, Langlotz F, Puls M, Murphy SB, Siebenrock KA (2007) Noninvasive three-dimensional assessment of femoroacetabular impingement. J Orthop Res 25(1):122–131
Tannast M, Siebenrock KA, Anderson SE (2007) Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol 188(6):1540–1552
Conflict of interest
The authors declare that no conflict of interest exists regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radetzki, F., Saul, B., Hagel, A. et al. Three-dimensional virtual simulation and evaluation of the femoroacetabular impingement based on “black bone” MRA. Arch Orthop Trauma Surg 135, 667–671 (2015). https://doi.org/10.1007/s00402-015-2185-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2185-y