Abstract
Background
Studies have shown that tranexamic acid reduces blood loss and transfusion need in patients undergoing total hip arthroplasty. However, no to date, no study has been large enough to determine definitively whether the drug is safe and effective. We examined whether intravenous tranexamic acid, when compared with placebo, was safe and effective in total hip arthroplasty.
Methods
The literature search was conducted using the PubMed, Cochrane Library, MEDLINE, EMBASE, and China National Knowledge Infrastructure (CNKI) databases. Data were evaluated using the generic evaluation tool designed by the Cochrane Bone, Joint and Muscle Trauma Group. Ultimately, 19 randomized controlled trials involving 1,030 patients were included.
Results
The use of tranexamic acid significantly reduced total blood loss by a mean of 305.27 mL [95 % confidence interval (CI) −397.66 to −212.89, p < 0.001], intraoperative blood loss by a mean of 86.33 mL(95 % CI −152.29 to −20.37, p = 0.01), postoperative blood loss by a mean of 176.79 mL (95 % CI −236.78 to −116.39, p < 0.001), and “hidden” blood loss by a mean of 152.70 mL (95 % CI −187.98 to −117.42, p < 0.001), resulting in a meaningful reduction in the proportion of patients requiring blood transfusion (odds ratio 0.28, 95 % CI 0.19 to 0.42, p < 0.001). There was no significant difference in occurrence of deep vein thrombosis, pulmonary embolism, or other complications among the study groups, or cost or hospitalization duration.
Conclusions
The data from this meta-analysis indicate that intravenous tranexamic acid may reduce blood loss and transfusion need in patients undergoing total hip arthroplasty without increasing the risk of complications. However, high-quality randomized controlled trials are required to validate the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty (THA) has long been considered the treatment of choice for osteoarthritis of the hip, developmental dysplasia of the hip, and osteonecrosis of the femoral head in older patients. In association with our aging society, the number of patients who will need THA may increase significantly in the next few years [1]. However, in THA, considerable blood loss remains a major problem, which can lead to a need for allogeneic blood transfusion. Such transfusion of allogeneic erythrocytes is not free of adverse events and has been associated with transmission of infectious diseases, increased postoperative bacterial infection, immune sensitization, transfusion-related acute lung injury, intravascular hemolysis, transfusion-induced coagulopathy, renal failure, admission to intensive care, and even death [2–4].
Several effective interventions have been developed to reduce blood loss and postoperative transfusion rates, such as preoperative autologous donation, cell salvage, controlled hypotension, regional anesthesia, and the use of erythropoietin and antifibrinolytics [5–7].
The antifibrinolytics include aprotinin, tranexamic acid (TXA), and ε-aminocaproic acid, which have different mechanisms of action [8]. TXA is a synthetic derivative of the amino acid lysine and a competitive inhibitor of plasminogen activation, and thus interferes with fibrinolysis. Compared with other antifibrinolytic drugs, TXA is cheaper and safer than aprotinin and more potent than the others. Numerous studies have evaluated the use of antifibrinolytics in orthopedic surgery and have shown them to be effective in reducing blood loss [9–24]. However, the available clinical trials and meta-analyses lack sufficient statistical power to determine the effectiveness of antifibrinolytic agents in total hip arthroplasty. Consequently, we performed a meta-analysis of randomized controlled trials from 1966 to November 2012 to investigate the evidence for the effectiveness and safety of intravenous TXA in relation to reducing blood loss and transfusion requirement in total hip arthroplasty.
Materials and methods
We performed our review according to the standards described in ‘Preferred reporting items for systematic reviews and meta-analyses’ statement [25].
Inclusion criteria
In this meta-analysis, we evaluated randomized controlled trials (RCTs) comparing TXA with control (placebo or nothing) in terms of hemorrhage and blood transfusion need during perioperative period. Studies for which full texts could not be obtained were excluded, as were articles that were not formally published or published only in abstract form in connection with meetings. Trials that compared TXA with another active intervention were excluded, except those with both a placebo group and the other intervention. Subgroup analyses of patients with different hemoglobin and hematocrit levels were performed. The subjects were adults who had undergone THA, regardless of the type or size of prosthesis or bone cement used. Studies that involved oral, injection of the articular cavity, or intramuscular treatment were excluded.
The intervention considered was the administration of intravenous TXA. Primary outcomes were estimated intraoperative, postoperative, total, and “hidden” blood loss, as well as allogeneic blood transfusion and changes in postoperative hemoglobin and hematocrit. “Hidden” blood loss, which is calculated as the total blood loss minus the measurable blood loss, usually includes blood extravasating into the tissues, remaining in the joint cavities, and lost via hemolysis. It is considered to be the main reason why the postoperative hemoglobin is lower than anticipated in joint replacement patients [26]. Secondary outcomes were the proportion of patients who had postoperative complications, especially deep vein thrombosis and pulmonary embolisms, as well as myocardial infarction and cerebrovascular accident. The economic effectiveness of TXA and hospitalization duration were also considered if data were available. When the data allowed, we also examined the dose–effect relationship between the dosage of TXA and the total blood loss, to explore the trends in the change in the relationship between TXA and blood loss.
Search strategy
For the review, we retrieved articles that were included in the MEDLINE, PubMed, EMBASE, Cochrane Library, and Chinese National Knowledge Infrastructure (CNKI) databases, which describe trials of TXA and THR and were published before November 2012, with no date restriction. Languages other than English and Chinese were excluded. For the electronic database search, the following exploded Medical Subject Headings (MeSH) terms were used: “Antifibrinolytics,” “Tranexamic acid,” “Cyklokapron,” “hip arthroplasty,” “hip replacement,” and “joint replacement.” The reference lists of related reviews and original articles identified were also reviewed for relevant trials, including clinical trials and randomized controlled trials, in adult humans.
Review methods
To select suitable references, three independent reviewers first applied the search strategy to scan the titles and abstracts from the databases to confirm that they fulfilled the inclusion criteria. When there was uncertainty about any of the vital information, the full article was retrieved for further scrutiny, or the authors of individual trials were contacted directly to provide further information, when necessary. To assess the methodological quality of the studies included, we used a modified version of the generic evaluation tool used by the Cochrane Bone, Joint and Muscle Trauma Group [27] (Table 1). The methodological quality of each trial was scored from 0 to 24, where higher scores indicated better quality. Disagreements were resolved by consensus or consultation with the senior reviewer. Data extracted from the included studies were entered independently by three reviewers. Any disagreement was resolved by the senior reviewer, LW. When this was not possible or data were missing through loss to follow-up, intention-to-treat principles were used.
Statistical analysis
Data were checked and entered into Review Manager 5.0 by the three reviewers independently and the results were cross-checked. Continuous data were entered as means and standard deviations (SDs), while dichotomous data were entered as number of events. The presence of heterogeneity was explored by Chi squared test with a significance set at a p value of 0.1, and the degree of heterogeneity was measured using the I 2 value [28]. The origins of heterogeneity, if present, were analyzed according to differences in methodological quality, characteristics of participants, and interventions. A non-significant Chi squared test result (a p value ≥0.1 and an I 2 value ≤50 %) suggested only a lack of evidence of heterogeneity; it did not necessarily indicate homogeneity, because the statistical power may have been insufficient to detect heterogeneity [29]. A fixed-effect model analysis was used to compare trials not showing heterogeneity, while a random-effects model analysis was used for those that did. For each study, the odds ratios (OR) and 95 % confidence intervals were calculated for dichotomous outcomes, and mean differences and 95 % confidence intervals for continuous outcomes. The relationship between TXA dosage and the total blood loss was explored using the SPSS software. The correlation coefficient (r) was used to evaluate the relationship between the dosage of TXA and total blood loss. p values less than 0.05 were considered to indicate significance.
Results
Results and patients’ characteristics
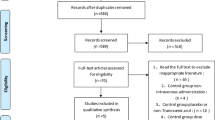
Following application and refinement of the literature search strategy, 19 independent randomized controlled trials [9–24, 30–32], containing 1,030 patients, were eligible for data extraction and meta-analysis (Fig. 1) (Table 2). Most were relatively well-designed and the quality assessment score of most was high, with a mode of 24, the highest possible score; a median value of 22; and a range of 14–24. The trials included were all of THA, and osteoarthritis was the most common diagnosis. Preoperative autologous blood donation or intraoperative clean blood collection were used in four studies [9, 13, 14, 22], and the amount of autotransfusion was not included in the postoperative blood transfusion statistics. Postoperative drainage was quantified between 24 and 48 h, when drains were in most cases removed. The study by Jamie [20], reported in 2011, included a group that received fibrin spray as well as TXA and control groups, was also included. The last study [24] compared four IV bolus methods. Since there were five randomized controlled groups in this study, we regarded it as four independent studies in the following comparative research (Table 3). Two studies compared the economic costs of blood products and TXA [11, 15] and favored the use of TXA. In addition to the key outcome measure below, some other adverse events are worth mentioning, which have been listed in Table 4.
Blood loss
Blood loss data were provided by all 19 studies. Seven studies [9, 13–15, 17, 31, 32] of a total of 382 patients, used an outcome of total blood loss. TXA significantly reduced total blood loss in the TXA group compared with the placebo group, by a mean of 305.27 mL (95 % CI −397.66 to −212.89 mL; p < 0.001; Fig. 2). Intraoperative blood loss volume was measured by weighing sponges and suction drainage at the end of surgery in nine studies [9, 13–15, 17–19, 22, 32]. The blood loss volume per patient was significantly less in the TXA group (weighted mean difference −86.33 mL (95 % CI −152.29 to −20.37 mL; p = 0.01; Fig. 3). Eight studies [9, 10, 13–15, 17, 22, 31], including 419 patients, provided postoperative blood loss data. The use of TXA significantly reduced postoperative blood loss, as measured by drainage volume, by a mean of 176.79 mL (95 % CI −236.78 to −116.79 mL; p < 0.001; Fig. 4). Two studies [15, 23], including 140 patients, had “hidden” blood loss data. The use of TXA significantly reduced “hidden” blood loss in the TXA group compared with the placebo group (weighted mean difference −152.70 mL (95 % CI −187.98 to −117.42 mL; p < 0.001; Fig. 5).
Changes in hemoglobin and hematocrit
Changes in postoperative hemoglobin (Hb) and hematocrit (Hct) were mentioned in nine studies [9, 13–15, 17–19, 30, 32]. However, data on changes in Hb and Hct with standard deviations were reported in only five [9, 13, 14, 17, 18]. The use of TXA significantly ameliorated the postoperative hemoglobin decrease, by 6.03 g/L in total (95 % CI 3.90 to 8.15 g/L; p < 0.001; Fig. 6) and the hematocrit decrease, by 2.29 % in total (95 % CI 1.42 to 3.16 %; p < 0.001; Fig. 7). We also conducted a subgroup analysis in chronological order, with 6-h, and 1- and 7-day changes in postoperative hemoglobin and 1- and 7-day changes in hematocrit. The Hct results of days 1 and 7 postoperatively were both significantly higher in the TXA group compared with the placebo group, and identical to the outcome for the total change in Hct; while the Hb results at 6 h and day-1 were significant, and identical to the outcome of the total change in Hb.
Allogeneic blood transfusion
Allogeneic blood transfusion data were provided by 18 studies [9–17, 19–24, 30–32]. The number of patients who required transfusion was significantly (28 %) less in the TXA group than the placebo group (95 % CI 0.19 to 0.42; p < 0.001; Fig. 8). Blood units transfused per patient data were available in only nine studies [9, 10, 14, 19, 20, 22–24, 31]. No significant evidence was available on reducing the average number of allogeneic red blood cell (RBC) transfusions per patient when the TXA group was compared with the placebo group. (weighted mean difference 0.3 units; 95 % CI −0.49 to 1.09 units; p = 0.45).
Thromboembolic complications
All 19 studies reported DVT complication data. Patients receiving TXA (539 patients) had 15 episodes of DVT, while the 535 patients who did not receive TXA had a total of 19 episodes. Thus, based on these numbers, DVT was not affected by the use of TXA (OR 0.73, 95 % CI 0.36–1.49, p = 0.39). Three trials [11, 24, 31] reported four pulmonary embolism events, three of 539 in the TXA group and one of 535 in the control group. However, there was no statistically significant difference in the risk of developing pulmonary embolism based on these data [OR 1.95; (95 % CI 0.34–11.06); p = 0.45].
Hospitalization duration
This outcome measure was reported in two studies [18, 19]. Hospitalization duration was not affected by the use of TXA when the TXA group was compared with the placebo group [weighted mean difference −0.58 days (95 % CI −1.6 to 0.43 days); p = 0.26].
Dose–effect relationship
This outcome was available in 11 studies [9, 10, 13–17, 22, 24, 30, 32], in which various doses of TXA were used. We plotted the TXA dose on the abscissa, and the corresponding total blood loss as the ordinate, to generate a scatterplot. In addition, the linear correlation coefficient (r) was also calculated. A significantly positive correlation between the dosage of TXA and total blood loss was found (r = −0.7258, p = 0.0033; Fig. 9). The total blood loss tended to decrease as the TXA dose increased.
Discussion
To minimize the possibility of bias, we performed a primary analysis including only RCTs, although some of them were not double-blinded. Our results showed that the use of TXA could significantly reduce total, postoperative, and “hidden” blood loss, as well as the number of patients who needed allogeneic transfusions. Comparing the data of total blood loss and dominant blood loss, it can be seen that “hidden” blood loss is an important issue in total hip arthroplasty. However, only two studies reported hidden blood loss measurement methods and results.
TXA also played a positive role in lowering the decrease of the postoperative hemoglobin and hematocrit. Furthermore, TXA did not increase the prevalence of deep vein thrombosis, pulmonary embolism, or hospitalization duration. These results are similar to the meta-analyses by Sukeik [29], although their studies differed from ours. However, the strength and reliability of the rate of adverse events is limited by the lack of a systematic investigation and the relatively short duration of postoperative observation.
Tranexamic acid, a synthetic derivative of the amino acid lysine, is an antifibrinolytic agent that acts by binding to plasminogen and blocking the interaction of plasmin (ogen) with fibrin, thereby preventing dissolution of the fibrin clot [33]. As a result, it contributes to reduced blood loss, as well as the number of blood transfusions per patient and the number of patients requiring transfusions when the tranexamic acid group was compared with the placebo group.
From this, one issue that must be addressed is whether TXA influences the fibrinolytic system in THA after the operation. The effects of TXA on the fibrinolytic system may means that it also affects prothrombin time, activated partial thromboplastin time, international normalized ratio, and platelets. However, only one trial [9] refers to changes in these postoperative indexes. During the perioperative period (up to day 1 postoperatively), prothrombin time, activated partial thromboplastin time, fibrinogen, and plasminogen-activator inhibitor showed no significant differences in the TXA group compared with the placebo group. Also, TXA may reduce blood loss by reducing induced fibrinolysis, as shown by a decreased D-dimer and increased PAP [9]. This also raises the issue as to whether TXA interacts with thromboprophylaxis agents. However, data are insufficient to investigate this further. Patients who received TXA did not have a higher risk of deep vein thrombosis or pulmonary embolism, compared with those who received placebo, based on the meta-analysis data. Nevertheless, randomized controlled trials with larger numbers of patients are needed to confirm the results of our meta-analysis.
In addition, the 19 studies included in our meta-analysis used various doses. When the effect estimates for a reduction in the total blood loss were plotted against the total amount of TXA administered, the best-fitting line showed a direct relationship between the amount of TXA given and the effect estimate. However, more data are needed to validate a relationship between dose and effect. From these results, the intravenous administration of 10–20 mg/kg (or 1 g) of TXA before the operation, followed or not, by 10–20 mg/kg 3–12 h afterwards, appears to be safe and effective. Benoni [30] and Norio [24] reported that intravenous administration of TXA only after the operation or before skin closure may weaken its effect in terms of reducing blood loss and allogeneic blood transfusion volume. Of course, this finding should also be confirmed by more data.
Our meta-analysis demonstrated the economic effectiveness and lack of an effect on hospitalization duration of TXA [11, 15, 18, 19]. Due to the high cost of allogeneic blood, TXA appears to have an advantage compared to transfusions. However, there was no significant effect on hospitalization duration in the TXA group compared with the placebo group.
The limitations of our study included insufficient data to support the analysis of functional outcome scores or quality of life outcome measures. In addition, the autotransfusion protocol, allogenic blood transfusion, different time periods of intravenous administration, surgical techniques, and measurement of blood loss likely contributed to the differences observed among studies. Furthermore, the trials included in our study were designed to assess the efficacy and safety of TXA in THA, after excluding high-risk factors such as patients with a history of cardiovascular disease, including angiocardiopathy, thromboembolic events, bleeding diathesis, allergy to TXA, renal failure, and bleeding diathesis, and those who were pregnant, or on warfarin or a therapeutic dose of low-molecular-weight heparin. In addition, with the exception of the total blood loss, our findings do not support the assumption that a higher dose of TXA necessarily reduces the intraoperative and postoperative blood loss or blood transfusion rates. Therefore, no definite conclusions regarding the safety and effectiveness of TXA can be derived from our meta-analysis regarding high-risk patients.
Conclusions
In conclusion, our analysis suggests that the use of TXA in THA significantly reduced the risk of bleeding and the need for allogeneic transfusion. However, data are insufficient to evaluate safety and cost-effectiveness. Thus, no definite conclusions regarding the clinical risk:benefit ratio of the use of tranexamic acid in THA can be drawn at this stage. Larger, high-quality prospective trials are required to strengthen our conclusions, define the optimal regimen, and assess the safety and cost-effectiveness of TXA before recommending its use in THA.
References
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785
Kumar A (2009) Perioperative management of anemia: limits of blood transfusion and alternatives to it. Clevel Clin J Med 76(Suppl 4):S112–S118
Lemaire R (2008) Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br 90:1128–1136
Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP (2003) Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 54:908–914
Cardone D, Klein AA (2009) Perioperative blood conservation. Eur J Anaesthesiol 26:722–729
Henry DA, Carless PA, Moxey AJ et al. (2002) Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev CD003602
Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA (2010) Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev CD001888
Dhawale AA, Shah SA, Sponseller PD et al (2012) Are antifibrinolytics helpful in decreasing blood loss and transfusions during spinal fusion surgery in children with cerebral palsy scoliosis? Spine 37:E549–E555
Ekback G, Axelsson K, Ryttberg L et al (2000) Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg 91:1124–1130
Ido K, Neo M, Asada Y et al (2000) Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg 120:518–520
Benoni G, Fredin H, Knebel R, Nilsson P (2001) Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand 72:442–448
Husted H, Blond L, Sonne-Holm S, Holm G, Jacobsen TW, Gebuhr P (2003) Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand 74:665–669
Lemay E, Guay J, Cote C, Roy A (2004) Tranexamic acid reduces the need for allogenic red blood cell transfusions in patients undergoing total hip replacement. Can J Anaesth 51:31–37
Yamasaki S, Masuhara K, Fuji T (2004) Tranexamic acid reduces blood loss after cementless total hip arthroplasty-prospective randomized study in 40 cases. Int Orthop 28:69–73
Johansson T, Pettersson LG, Lisander B (2005) Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop 76:314–319
Niskanen RO, Korkala OL (2005) Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double-blind study of 39 patients with osteoarthritis. Acta Orthop 76:829–832
Claeys MA, Vermeersch N, Haentjens P (2007) Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg 107:397–401
Kazemi SM, Mosaffa F, Eajazi A et al (2010) The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics 33:17
Singh J, Ballal MS, Mitchell P, Denn PG (2010) Effects of tranexamic acid on blood loss during total hip arthroplasty. J Orthop Surg 18:282–286
McConnell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AW (2011) Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. Acta Orthop 82:660–663
Malhotra R, Kumar V, Garg B (2010) The use of tranexamic acid to reduce blood loss in primary cementless total hip arthroplasty. Eur J Orthop Surg Traumatol 21:101–104
Clave A, Fazilleau F, Dumser D, Lacroix J (2012) Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a case-control study in 70 patients. Orthop Traumatol Surg Res 98:484–490
Hai FZZJY (2012) Effect of tranexamic acid on the hidden blood loss after total hip arthroplasty. J Chongqing Med Univ 37(4):359
Imai N, Dohmae Y, Suda K, Miyasaka D, Ito T, Endo N (2012) Tranexamic Acid for reduction of blood loss during total hip arthroplasty. J Arthroplasty 27:1838–1843
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Sehat KR, Evans RL, Newman JH (2004) Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 86:561–565
Handoll HH, Gillespie WJ, Gillespie LD, Madhok R (2007) Moving towards evidence-based healthcare for musculoskeletal injuries: featuring the work of the Cochrane Bone, joint and Muscle Trauma Group. J R Soc Promot Health 127:168–173
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Sukeik M, Alshryda S, Haddad FS, Mason JM (2011) Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br 93:39–46
Benoni G, Lethagen S, Nilsson P, Fredin H (2000) Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta Orthop Scand 71:250–254
Garneti N, Field J (2004) Bone bleeding during total hip arthroplasty after administration of tranexamic acid. J Arthroplasty 19:488–492
Rajesparan K, Biant LC, Ahmad M, Field RE (2009) The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br 91:776–783
McCormack PL (2012) Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 72:585–617
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Xd., Tao, Lj., Li, J. et al. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg 133, 1017–1027 (2013). https://doi.org/10.1007/s00402-013-1761-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-013-1761-2