Abstract
Introduction
Degenerative articular disc perforations of the triangular fibrocartilage (TFC) of the wrist are characterized by fibrocartilage cell loss and are often associated with ulna-plus situations. Apoptosis has been found to play a crucial role in fibrocartilage cell loss, however, the molecular mechanism and mediators are still poorly understood.
Aim
The purpose of this study was to identify receptors to apoptosis in degenerative disc lesions.
Patients
Included in the study were 17 patients with degenerative articular disc tears of the TFC (Palmer type 2C). Following arthroscopic debridement of the TFC, histological sections were examined to assess the presence of apoptosis. Apoptosis was determined using TRAIL and death receptor DR4 agonists for immunohistochemical analyses. The number of cells positive for apoptosis was then correlated with ulna length.
Results
Cells positive for TRAIL and DR4 were found in all specimens. The number of cells positive for TRAIL was significantly increased in specimens of patients with an ulna positive variance (P = 0.040). However, DR4 was not significantly increased in ulna plus (P > 0.05). Both, TRAIL and DR4 positive cells were found to be evenly distributed throughout each specimen. There was no accumulation of any type of cells in any particular zone of the biopsies.
Conclusion
This is the first study that shows that TFCC cells express TRAIL and DR4, which suggests that apoptosis, as well as, mechanical trauma are involved in the development of disc perforation. The TRAIL/DR4 receptor system is a molecular mediator of apoptosis induction in TFC cells and therefore plays a role in cell loss in degenerative disc lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulnar impaction syndrome is thought to be due to chronic, excessive loading of the ulno-carpal joint, which may cause degenerative lesions of the triangular fibrocartilage complex (TFCC) [1]. The syndrome is characterized by ulnar-sided wrist pain, reduced range of motion, and grip strength. It has been shown that patients with ulna neutral or ulna negative variance exhibit fewer degenerative changes than those with ulna positive variance [2]. Equally, the loss of tissue cellularity contributes to disc degeneration [3]. Most importantly, recent studies have shown that apoptosis plays a crucial role in the occurrence of cell loss in traumatic Palmer 1A lesions [4], as well as, degenerative lesions of the TFC [5, 6].
The extrinsic and/or intrinsic pathway may induce apoptosis. In 1995, Wiley et al. identified a novel TNF-related apoptosis-inducing ligand, TRAIL (Fig. 1) [7]. TRAIL was capable of inducing apoptosis by binding a cell-bound apoptosis inducing receptor.
Simplified extrinsic pathway: Binding of molecules such as, TNF, FAS or TRAIL leads to the recruitment of several other molecules in the intracellular “death domain” of the receptor. After a “death inducing signalling complex” (DISC) is formed, it is able to transform caspases into their active form, which results in apoptosis
The extrinsic pathway is initiated by activation of transmembrane receptors of the TNF superfamily, which are also called “death receptors”. Binding of molecules, such as, TNF, FAS (Fibroblast Associated ligand) or TRAIL leads to the recruitment of several molecules in the intracellular “death domain” of the receptor. Most intracellular receptors are of the FADD (Fas Associating protein with Death Domain), and the TRADD family (TNFRSF1A-associated via Death Domain) (Fig. 1). Via these mechanisms the “death inducing signalling complex” (DISC) is formed. This complex is able to transform caspases into their active form. TRAIL and its death inducing receptors (DR) are usually known to induce apoptosis in tumor cells. Recent studies, however, were able to show that TRAIL is also involved in apoptotic processes in degenerative fibrocartilage tissue [8]. TRAIL’s influence on fibrocartilage death may be a factor in extrinsically induced apoptosis [8].
Studies on degenerative human intervertebral discs suggest that the expression of TRAIL correlates with the rate of cartilage degeneration [8, 9]. Knowledge of the particular apoptotic pathways involved in the degeneration process will enable effective treatment in the future.
In contrast, the intrinsic pathway is initiated mainly by mitochondrial stress. This triggers the release of cytochrome c, which together with other molecules, forms the apoptosome cluster. Thus, caspases are activated and the cell will undergo cell death by DNA fragmentation [10].
To date, very little is known about the ligands and their receptors underlying apoptotic cell loss in the TFC, and whether or not ulna length is also a causative factor [5, 6]. The purpose of this study was to identify receptors of apoptosis in degenerative disc lesions. Furthermore, we investigated the role of ulna length by correlating it with the number of receptor positive cells. We hypothesized that the number of receptor positive cells is correlated with ulna length.
Patients and methods
Patients
Our institution’s ethical committee approved the experimental study design. All participating 17 patients (11 males, 6 females) were diagnosed with a Palmer type 2C lesion during arthroscopy. From our study, we excluded patients with lesions other than type 2C. In addition, we excluded patients with other, additional wrist disorders, such as: radiocarpal arthritis, previous intra-articular fracture of the distal radius or carpus. A previous reconstruction of the wrist also constituted an exclusion criterium. Prior to arthroscopy, all patients received a plain biplanar radiographic examination. Static and dynamic ulnar variance was determined from the perpendicular to the forearm axis at the subchondral distal edge of the sigmoid fossa on the radius. Ulnar positive was defined as the radius exceeding the perpendicular plane by more than 0.5 mm. Ulnar negative was defined as the radius undercutting the perpendicular plane below 0.5 mm.
Subsequently, we assigned patients to two different groups based on patient’s ulna length: one group consisted of patients with an ulnar positive variance group A (UPV); the other group included patients with ulnar negative or neutral variance group B (UNV). Group A’s average ulna length measured 2.4 ± 0.3 mm (range 2.0–3.1 mm), group B’s was 0.7 ± 0.5 mm (range 0.1–1.2 mm). A degenerative perforation located in the articular disc of the TFCC was found in all patients. This perforation was associated with underlying ulna head chondromalacia (n = 9). All patients suffered from either painful ulnar grinding or exhibited a positive fovea sign. This pain was further exacerbated when the wrist was deviated against resistance towards the ulna. The distal radio-ulnar joint was stable in all patients. Table 1 details the demographics of the two groups. Three patients were not able to recall the time of onset of their ulno-carpal symptoms.
Prior to the arthroscopic procedures, all patients had undergone a conservative treatment regimen consisting of a brace worn for 1–3 weeks, and anti-inflammatory medications. Conservative treatment, however, did not yield any improvement of the ulno-carpal symptoms in any of the patients.
Surgical technique
The 3–4 portal was used to introduce the arthroscope (2.4 mm) while the wrist was distended. For débridement, the biopsy punch was introduced into the 4–5 portal, or the 6R portal. We ensured the capture of all degenerative tissue by routinely resecting sufficiently far into the disc. The biopsy punch to be of a 3 mm diameter (Arthrex, Munich, Germany). A shaver was employed for the débridement. Following the procedure, the wrist was rinsed multiple times to remove all débrided tissue. Patients then wore a short arm cast for a period of 2 weeks.
Immunohistochemistry
The tissue was fixed for 3 days in a 4% formalin solution, and subsequently embedded in paraffin. Axial sections of the specimens were prepared. Their thickness measured 3–4 μm and death receptor DR 4 were detected by use of monoclonal antibodies to and DR 4 (Santa Cruz, Biotechnology, CA, USA). Paraffin sections were de-waxed in Xem-200 and subsequently re-hydrated. No antigen retrieval was necessary. The primary antibody dilution was 1:50 for, and 1:100 for DR 4 in 3% BSA. Secondary antibodies for rabbit anti-goat were diluted 1:500 in 3% BSA (Linaris GmbH, Wertheim, Germany). For the staining we employed the ABC-AP Staining System (Linaris GmbH, Wertheim, Germany) closely following manufacturer’s protocol. All slides were counterstained with haematoxylin and mounted under cover-slips using Aquatex.
Analysis
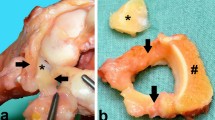
Three biopsies were taken of each patient’s disc. Each specimen was divided into three zones. In a low magnification the biopsies were examined under the microscope to determine inner, middle, and outer zones. The inner zone has rectangular edges, while the outer zone can be characterized by its round edges. According to these criteria, the biopsy was divided into three parts (Fig. 2). Each zone’s total number of cells (hemalaun), positive for group A (UPV) TRAIL, and group B (UNV) DR-4 were recorded by an independent physician. While the counting was performed, the microscope was being set to three high power fields (HPF) (100×). Then we determined the percentage of positive cells for each marker per zone. The number of apoptotic cells was correlated with ulna length.
Statistical analysis
For the statistical analysis the Mann–Whitney U test and Wilcoxon test were used. Additionally, the Spearman Rank Correlation test was used for correlating time of trauma and frequency of apoptosis. The analyses were performed using SPSS® V.11.0 for Windows (SPSS Inc., Chicago, USA) and SAS 9.1 for Windows (Version 9.1; SAS Institute Inc., NC, USA). Statistical significance was accepted at P < 0.05.
Results
TRAIL
Cells positive for TRAIL could be detected in all specimens (Fig. 3a). In comparison with group B (UNV), group A (UPV) exhibited a significantly increased number of cells positive for TRAIL (P = 0.04).
DR4
Cells positive for Fas-ligand were found in all specimens (Fig. 3b). Comparing the number of positive cells between group A (UPV) and B (UNV), no statistical difference could be found (P > 0.05).
All apoptotic cells, which were positive for TRAIL and DR4, were found to be evenly distributed throughout each specimen. There was no accumulation of cells in any particular zone. No significant differences could be found between in inner, middle and outer regions of the biopsies (P > 0.05). In addition, there was no correlation between time of trauma, patient age or gender and the number of apoptotic cells. The average number of apoptotic cells per zone is detailed in Table 2.
Discussion
The study could detect TRAIL/DR4 positive cells in degenerative disc lesions. Unglaub et al. found that apoptosis takes place via the extrinsic and the intrinsic apoptotic pathways [6]. This study suggests that TRAIL and DR4 induce apoptosis in degenerative disc lesions. Overall, these results indicate that apoptosis plays a major role in the development of degenerative disc lesions, and that TRAIL is more prevalent in patients with ulna positive variance.
Tatebe et al. could show in their study that spontaneous repair of the central lesion of the TFCC occurred in 16 of 32 wrists (50%) following an ulnar shortening procedure [11]. This could be confirmed by a second-look arthroscopy performed after the ulnar-shortening procedure. Furthermore, histological examinations of the biopsy specimens showed that regenerated tissue contained both, fibrous connective tissue, as well as, fibrocartilaginous components. These findings contradict the results of Bednar who believed the central, avascular part of the disc to have no healing potential [12]. Knowledge of the exact apoptotic pathways and the mechanisms that are able to trigger regeneration could lead to more effective treatments.
Unglaub et al. could show that apoptosis plays an important role in degenerative disc lesions associated with ulna positive variance [5, 6].
In addition, it is known that the peripheral 10–40% of the articular disc are well-vascularized [12, 14]. Peripheral lesions are repaired by suture due to their good vascularity and healing potential, as shown by some studies [13, 15, 16]. However, the central portion of the TFC is avascular [12]. We speculate that the cells of this particular part are more vulnerable to apoptosis because of their avascular situation [12]. Because it is not standard procedure to debride in the periphery, we could not obtain biopsies from this particular region to prove this point.
The pharmacological manipulation of apoptosis has clinical relevance and is a new frontier in the development of novel approaches to treat human diseases [17, 18]. Furthermore, an efficient therapy approach that would directly address and neutralize the apoptosis-inducing ligand TRAIL might help to at least delay progression of disc degeneration in the wrist. A comparable approach that involves Fas ligand function perturbing antibodies has already shown promising results in the regeneration and functional recovery following spinal cord injuries in an animal model [19].
In animal models it was shown that intra-articular injections containing apoptosis inhibitors (Z-VAD-fmk) slow the rate of mechanically induced chondrocyte apoptosis. The injections were able to prevent long-term chondrocyte apoptotic cell loss and thereby contained the progression of cartilage degeneration [20–22].
Studies on cells of rat intervertebral discs showed that caspase 8 inhibitors have an anti-apoptotic effect. However, caspase 9 inhibitors did not show this property. This suggests that the extrinsic apoptotic pathway plays a role in fibrocartilage degeneration. TRAIL and FAS are ligands involved in mediating the extrinsic pathway [23].
Another animal model showed that TNF-alpha inhibitors have beneficial effects on the rate of apoptosis in intervertebral discs. Because TRAIL is closely related with TNF-alpha, it may have promising therapeutic relevance [24].
Human cartilage cell lines also showed that it is possible to sufficiently inhibit caspase 3, while also retaining the chondrocytic characteristics, such as extracellular matrix production [25]. To avoid potentially toxic side-effects, it is desirable to know the particular apoptotic pathway leading to the chondrocyte loss. This way, only very specific apoptotic inhibitors would be employed.
References
Palmer AK (1989) Triangular fibrocartilage complex lesions: a classification. J Hand Surg [Am] 14(4):594–606
Palmer AK, Werner FW (1981) The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg [Am] 6(2):153–162
Mikic ZD (1989) Detailed anatomy of the articular disc of the distal radioulnar joint. Clin Orthop Relat Res 245:123–132
Unglaub F, Fellenberg J, Germann G, Bickert B, Sauerbier M, Richter W (2007) Detection of apoptotic cartilage cells in symptomatic central tears of the triangular fibrocartilage. J Hand Surg [Am] 32(5):618–622
Unglaub F, Wolf MB, Thome MA, Germann G, Sauerbier M, Reiter A (2008) Correlation of ulnar length and apoptotic cell death in degenerative lesions of the triangular fibrocartilage. Arthroscopy 24(3):299–304
Unglaub F, Thomas S, Kroeber M, Dragu A, Fellenberg J, Wolf MB et al (2009) Apoptotic pathways in degenerative disc lesions in the wrist. Arthroscopy 25(12):1380–1386
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3(6):673–682
Bertram H, Nerlich A, Omlor G, Geiger F, Zimmermann G, Fellenberg J (2009) Expression of TRAIL and the death receptors DR4 and DR5 correlates with progression of degeneration in human intervertebral disks. Mod Pathol 22(7):895–905
Zhang L, Niu T, Yang SY, Lu Z, Chen B (2008) The occurrence and regional distribution of DR4 on herniated disc cells: a potential apoptosis pathway in lumbar intervertebral disc. Spine (Phila Pa 1976) 33(4):422–427
Chen B, Fellenberg J, Wang H, Carstens C, Richter W (2005) Occurrence and regional distribution of apoptosis in scoliotic discs. Spine 30(5):519–524
Tatebe M, Horii E, Nakao E, Shinohara T, Imaeda T, Nakamura R et al (2007) Repair of the triangular fibrocartilage complex after ulnar-shortening osteotomy: second-look arthroscopy. J Hand Surg [Am] 32(4):445–449
Bednar MS, Arnoczky SP, Weiland AJ (1991) The microvasculature of the triangular fibrocartilage complex: its clinical significance. J Hand Surg [Am] 16(6):1101–1105
Wolf MB, Kroeber MW, Reiter A, Thomas SB, Hahn P, Horch RE et al (2008) Ulnar shortening after TFCC suture repair of Palmer type 1B lesions. Arch Orthop Trauma Surg [Epub ahead of print]
Unglaub F, Kroeber MW, Thomas SB, Wolf MB, Arkudas A, Dragu A et al (2009) Incidence and distribution of blood vessels in punch biopsies of Palmer 1A disc lesions in the wrist. Arch Orthop Trauma Surg 129(5):631–634
Estrella EP, Hung LK, Ho PC, Tse WL (2007) Arthroscopic repair of triangular fibrocartilage complex tears. Arthroscopy 23(7):729–737
Reiter A, Wolf MB, Schmid U, Frigge A, Dreyhaupt J, Hahn P et al (2008) Arthroscopic repair of Palmer 1B triangular fibrocartilage complex tears. Arthroscopy 24(11):1244–1250
Lavrik IN, Golks A, Krammer PH (2005) Caspases: pharmacological manipulation of cell death. J Clin Invest 115(10):2665–2672
Green DR, Kroemer G (2005) Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest 115(10):2610–2617
Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C et al (2004) Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med 10(4):389–395
Dang AC, Warren AP, Kim HT (2006) Beneficial effects of intra-articular caspase inhibition therapy following osteochondral injury. Osteoarthritis Cartilage 14(6):526–532
D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M (2006) Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum 54(6):1814–1821
Costouros JG, Kim HT (2007) Preventing chondrocyte programmed cell death caused by iatrogenic injury. Knee 14(2):107–111
Park JB, Park IC, Park SJ, Jin HO, Lee JK, Riew KD (2006) Anti-apoptotic effects of caspase inhibitors on rat intervertebral disc cells. J Bone Joint Surg Am 88(4):771–779
Xu R, Shao Z, Xiong L (2008) Experimental study on inhibitory effect of niacinamide on tumor necrosis factor-alpha-induced matrix degradation of annulus fibrous tissue in vitro. J Huazhong Univ Sci Technolog Med Sci 28(5):576–579
Nuttall ME, Nadeau DP, Fisher PW, Wang F, Keller PM, DeWolf WE Jr et al (2000) Inhibition of caspase-3-like activity prevents apoptosis while retaining functionality of human chondrocytes in vitro. J Orthop Res 18(3):356–363
Acknowledgments
The study was supported by the ELAN Fonds, University of Erlangen, Germany.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Unglaub and S. B. Thomas contributed equally to this work.
Rights and permissions
About this article
Cite this article
Unglaub, F., Thomas, S.B., Kroeber, M.W. et al. Expression of TRAIL and death receptor DR4 in Palmer type 2 TFCC lesions. Arch Orthop Trauma Surg 130, 1215–1220 (2010). https://doi.org/10.1007/s00402-009-0988-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-009-0988-4