Abstract
Autism is a neurodevelopmental disorder characterized by deficits in social interaction and social communication, as well as by the presence of repetitive and stereotyped behaviors and interests. Brodmann areas 44 and 45 in the inferior frontal cortex, which are involved in language processing, imitation function, and sociality processing networks, have been implicated in this complex disorder. Using a stereologic approach, this study aims to explore the presence of neuropathological differences in areas 44 and 45 in patients with autism compared to age- and hemisphere-matched controls. Based on previous evidence in the fusiform gyrus, we expected to find a decrease in the number and size of pyramidal neurons as well as an increase in volume of layers III, V, and VI in patients with autism. We observed significantly smaller pyramidal neurons in patients with autism compared to controls, although there was no difference in pyramidal neuron numbers or layer volumes. The reduced pyramidal neuron size suggests that a certain degree of dysfunction of areas 44 and 45 plays a role in the pathology of autism. Our results also support previous studies that have shown specific cellular neuropathology in autism with regionally specific reduction in neuron size, and provide further evidence for the possible involvement of the mirror neuron system, as well as impairment of neuronal networks relevant to communication and social behaviors, in this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (hereafter referred to as autism) include brain developmental conditions of unknown etiology typically diagnosed in children by 3 years of age [59]. The diagnosis is made upon the presence of poor social interaction and communication skills as well as restricted and repetitive patterns of behavior, interests, or activities [1]. Although autism is pervasive in our society, affecting approximately 1 in 110 children with a gender ratio of 4.5 boys to every girl, its etiology still remains frustratingly unclear. This is mainly due to the highly heterogeneous clinical expression, with some children excelling in specific niches while over a third have an intellectual quotient that falls to 70 points [59]. Despite the broad range of severity, determining the basis of social impairment may shed light on a common ground among autism’s diverse causes.
As one of the core symptoms of autism is a deficit in social communication, an important area to assess at the cellular level would be one that is implicated in this function. In the left hemisphere, Brodmann areas 44 and 45 have a well-established role in syntactic processing and language production and more recently in processing emotional behavior and empathy [7, 53, 72], skills that are necessary for communication. In the right hemisphere, this region is activated during processing prosody, metaphoric sentence judgements, fine motor control, salient cue detection, and response inhibition [6, 31, 72], which provide information to respond appropriately in a social situation.
Another core symptom is poor interaction with family members starting at young age. This lack of reciprocal social contact affects the child’s ability to develop skills that are crucial in social development, particularly imitation [62]. These are skills that are glaringly absent in children with autism, who display inadequate performance on tasks that demand theory of mind (ToM), the ability to understand and replicate other’s mental states [5, 22, 32, 49]. This aptitude for empathizing with someone else’s mindset and predicting his or her subsequent behavior develops in the early years of life, potentially emanating from imitation, and is essential for social interactions [3, 58]. In a recent study, Senju and colleagues measured ToM ability in high-functioning patients with autism to assess their ability to recognize that the world-view of themselves and others can be different [67]. Patients with autism were able to use other cognitive resources to determine someone else’s world-view when prompted, but did not do so naturally, indicating impairment in ToM. While many studies have shown this disruption in ToM in patients with autism, the reason it does not develop properly remains unknown. One hypothesis for the lack of ToM is the dysfunction in the mirror neuron system early in childhood, which would likely cause a cascade of social impairments that characterize autism [23, 79].
Mirror neurons were first detected in the macaque premotor area F5 [24] and the inferior parietal cortex [20] as neocortical neurons that fired both when a macaque monkey observed an action and completed that action himself [9, 17, 24, 60]. Because of their specialized activity, mirror neurons were thought to play a key role for encoding imitation. Despite some controversy [71], evidence supports the existence of a homolog system in humans [40, 61, 79]. Functional magnetic resonance imaging contributions determined that areas 44 and 45 of the posterior inferior frontal cortex have an essential role in imitation, and therefore may be involved in the mirror neuron system [27, 39, 40, 42, 61]. As previously mentioned, impairment in the mirror neuron system is thought to be directly related to social deficits in autism [78, 79]. Many studies have disproven a dysfunction in mirror neurons through tasks that involve movement imitation (e.g. [30, 70]). While these results do show that patients with autism have appropriate responses in motor mimicking, they leave the possibility that brain function necessary for social tasks involving imitation may still be abnormal.
The link between the pathology of autism and impairment in the mirror neuron system remains controversial. The major problem is that only approximately 10 % of neurons are thought to be mirror neurons in areas 44 and 45 [24]. Magnetoencephalography and electroencephalography studies are not able to localize the area that shows electrical changes in brain [29]. Functional magnetic resonance imaging studies permit the localization of brain regions that are activated during a given task, and while this can show which parts of the brain are activated differently in autism, it is not specific enough as the tasks typically activate not only regions involved in imitation but motor and visual cortices as well, and possibly regions that process emotion. Also, as mirror neurons are likely a small percentage of neurons in certain brain areas, it is difficult to determine if the activated or non-activated regions actually represent the involvement of mirror neurons to any specific extent. For these reasons, it is important to analyze the brains of patients with autism at the cellular level to determine whether there are abnormalities present.
To address this need, Coleman and colleagues carried out a preliminary non-stereologic study of area 44 comparing a 21-year-old patient with autism and two normal subjects, aged 18- and 25-years [12]. They found no significant difference in the density of pyramidal neurons, other neurons, and glia, but emphasized the limitations of their study due to the large interindividual variability. To explore further this region, we obtained stereological estimates of neuron size and numbers as well as regional volume in areas 44 and 45 in a larger sample of patients with autism and age- and hemisphere-matched controls.
Materials and methods
Subjects
A total of eight postmortem brains obtained from patients with autism were analyzed (one hemisphere per case) in comparison to age- and hemisphere-matched controls (age range 4–66 years; see Table 1 for details). The patients spanned most developmental time points, although no age-defined subgroups were considered because of the small sample size. The sample consisted of three left hemisphere and five right hemisphere pairs. For two controls (ages 4 and 25), each hemisphere was used to match two cases. The right hemisphere of the 4-year-old served as a control for the 5-year-old patient with autism and the left hemisphere for the 4-year-old patient with autism; the right hemisphere of the 25-year-old was a control for the 21-year-old and the left hemisphere for the 32-year-old.
All subjects with autism were identified based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) and the Autism Diagnostic Interview (ADI-R). None of the cases demonstrated chromosomal abnormalities. Autopsies were performed after obtaining the informed consent from a relative and approval from the relevant institutional review boards. Demographic and clinical data are shown in Table 1.
Tissue processing
The tissue processing was performed at the New York State Institute for Basic Research in Developmental Disabilities (IBR) in Staten Island, NY, USA, and at the Morphologic Brain Research Unit (UWMBRU) at the University of Wuerzburg, Germany [56, 73, 76]. The brains were divided mediosagittally and all but two cases had only one hemisphere available for study (Table 1). The brains underwent immersion-fixation in 10 % formalin for at least 3 months and were then embedded in celloidin. Hemispheres processed at IBR were cut into a complete series of 200-μm thick coronal sections [37], and hemispheres processed at UWMBRU were cut into a complete series of 500-μm thick coronal sections. Each third section was available for staining in the 200-μm thick series, and every other section was available in the 500-μm thick series. All selected sections were stained with gallocyanin, mounted, and coverslipped at UWMBRU [38].
Brain regions and cytoarchitecture
Areas 44 and 45 constitute the opercular and triangular parts of the posterior inferior frontal gyrus. We defined the borders of these areas using Brodmann’s classification: caudally with premotor area 6, separated by the precentral sulcus; rostrally by area 46, separated with the ascending ramus of the Sylvian fissure; dorso-caudally by area 9, and dorso-rostrally by area 46, separated by the inferior frontal sulcus; and ventrally by the orbital part of the premotor area, area 47, and the insula [8]. The distribution of these areas is shown visually on the lateral view of the brain in Fig. 1.
Area 6 is an agranular region in which the layer separation is less distinct than in areas 44 and 45. In area 6, layer IV is less discrete from surrounding pyramidal layers III and V, and the cells in this area are radially organized; in contrast, our region of interest has a more distinct granularity and the cellular organization becomes columnar instead of radial [8, 75].
Areas 9 and 46 are both granular regions in which the six layers are clearly distinguishable. Layers II and IV are clearly demarcated from the rest of the cortical layers by their granule cells. Layer III can be divided into a superficial part (IIIa), which is sparsely populated with small pyramidal cells, and IIIb-c, which shows a denser presence of larger pyramidal neurons. Layer V has the opposite gradient with sublayer Va being denser and Vb comparatively sparser. The border between layer VI and adjacent layer Vb and the white matter on either side is sharp because of the density of layer VI. To distinguish areas 9 and 46 from our region of interest, we looked for a columnar cellular organization, a more robust gradient in layer III density, and large pyramidal neurons of layers III and V encroaching on layer IV [8, 75]. The difference between these areas is depicted in Fig. 2.
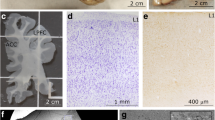
a Right hemisphere human brain section stained with gallocyanin showing cortical areas 44, 45, 46, and the frontoinsular cortex (FI), and photomicrographs showing the cytoarchitectural characteristics of area 45 (b) as well as neighboring area 6 (c), area 46 (d), and insula (e). Scale bar 2 cm (a) and 200 μm (b–e); cc corpus callosum, ic internal capsule
Ventrally, the insula forms an important neighboring area to the medial and posterior parts of areas 44 and 45. Along with the distinct change in cytoarchitecture to an agranular cortex, the presence of the band-like layer V of the insula as well as the claustrum in the adjacent white matter provides good landmarks for the insula. The insular cortex, in comparison to areas 44 and 45, has a thick layer I, a less dense layer IV, and an indistinct border between layer VI and the white matter. Globally, the most striking differences between these areas are the loss of distinct granularity and loss of columnar organization (Fig. 2). In comparison, adjacent area 47 shows an apparently lighter layer III as the density of pyramidal neurons decreases, adopting a patchy, clustering distribution. Other distinctive features of area 47 are a more distinguishable layer IV and a less perceptible columnar organization [75].
Apart from demarcating our areas of interest from adjacent areas, we also established histological criteria to define layers within areas 44 and 45, which are six-layered dysgranular areas. Layer I is a sparse, virtually acellular layer. Layer II is a granular layer, denser than any other layer in the frontal lobe, and thus is clearly distinguishable. Layer III is subdivided into three sublayers: IIIa–c. The sublayers have larger pyramidal neurons as they approach layer IV. Layer IV is a dense granular layer, overrun by the both adjacent layers III and V. Layer V is subdivided into Va, a denser sublayer with large pyramidal neurons, and Vb, a sparser sublayer with fewer pyramidal neurons. Layer VI is a dense layer populated with interneurons and pyramidal neurons, well demarcated from the white matter ([2, 8, 68, 75]—see Fig. 2 for visualization).
The border between layer II, which is characteristically a dense layer with small neurons, and IIIa, a sparser layer with pyramidal neurons, is well defined by the change in cell density and size. However, neurons in layers III and V typically overrun layer IV, making the delineation of layer IV ambiguous. To maintain consistency in our parameters through all our cases, we chose uniformity over accuracy: if the borders of layer IV were not clearly visible, the upper boundary of layer III and the lower boundary of layer V were placed in the middle of layer IV. The border between layer V and VI is typically very clear: layer Vb is sparse and has pyramidal neurons, whereas layer VI is dense with interneurons and neurons.
Stereologic design
For stereologic quantification, we selected every 15th section in the 200-μm thick series and every 4th section in the 500-μm thick series within the range of sections containing areas 44 and 45 for each case. This approach ensured that areas 44 and 45 were sampled in a consistent and unbiased manner with a number of sections that ranged between 4 and 12, depending on the section thickness. The quantitative analysis was performed using a stereology workstation equipped with a Zeiss Imager A1 microscope, Plan-Neofluar 2.5× (N.A. 0.075) and 40× (N.A. 0.75) objectives, a motorized stage (Ludl Electronics, Hawthorne, NY, USA), a MBF CX9000 camera (MBF Bioscience, Williston, VT, USA), and stereology software (StereoInvestigator version 10.2; MBF Bioscience).
Starting with a random section number, a systematic-random sampling scheme was applied throughout areas 44 and 45. The boundaries of layers III, V, and VI were defined at low magnification (2.5× objective) on the computer display using the cytoarchitectural characterization described above. The Optical Fractionator probe was used to calculate an estimate of the total number of neurons, as we did not count neurons through the entire thickness of the tissue or each section available of our region of interest [65, 77]. For some cases, the mounted thickness of these materials had already been estimated by other collaborators [64, 73], but in cases that had not yet been used we calculated the mounted thickness by averaging the measurements of a random sampling of 10 sites per slide with the 40× objective in areas 44 and 45. As a main focus of our study involved identifying and measuring pyramidal neuron number and size, we had several criteria that defined an object as a pyramidal neuron: the typical triangular shape, visible apical dendrite, and a clear large nucleus with a dark nucleolus (Fig. 3b, d).
For optimal estimation of neuronal populations, the dimensions of the sampling grid were set to sample at least 150 pyramidal neurons per case [65]. The software defined a systematic-random sampling sequence of counting frames and grids within the outlines of each layer of areas 44 and 45, in which pyramidal neurons were quantified. The quantification of pyramidal neurons was done at high magnification (40× objective) on the computer display using the characteristics described before. A disector height of 20 μm was set in order to ensure that quantification only occurred in the area of the tissue in which there was optimal staining. Guard zones of 2 μm were used on the top and bottom of the disector of every section in order to prevent biases related to sectioning artifacts. The parameters used for Optical Fractionator are summarized in Table 2.
The total volume of each analyzed layer in areas 44 and 45 was estimated using the Cavalieri probe through the measurement of the cross-sectional area of layers III, V, and VI. Along with the quantification of pyramidal neurons, their perikaryal volume was also measured using the Nucleator probe. To measure neuron volume, the user marks the center of the neuron and the probe extends a user-defined number of rays out of the center marker. The user then identifies the intersection of the rays with the edges of the neuron, and the program calculates the neuronal volume. Due to the typical shape of the pyramidal neurons and the 2D volume estimation calculation by the Nucleator, we chose to use six rays to precisely estimate the neuron’s shape. Because their size is variable, all pyramidal neurons that were counted were also measured for volume estimation.
When we initiated this study, we recounted four cases randomly selected from the series to assess the consistency of our parameters, and obtained an average difference of 17 % between these recounts. This seemingly high variability was somewhat unexpected based on our past analyses of the same materials [64, 73] and turned out to be generated by the actual convolution of the gyral patterns in the inferior frontal region among these cases. In fact, previous studies of the architecture of this region did show a certain degree of interindividual variability in gyral and sulcal anatomy [2, 46, 55, 68]. The average difference for cases that were sectioned optimally for our region of interest had a difference of less than 10 % and was adequate for precise stereologic estimates; however, differences between recounts were higher in two of these cases (a control child and an adult with autism) due to unavoidable variability in the angle of section, the brains having been processed whole in a strict coronal plane. This can at times generate tangential section planes through the cortical layers and render the precise determination of regional borders more difficult, a problem that commonly arises in quantitative neuropathologic studies using stereology. We had to adopt a conservative approach in these two cases, by limiting the analysis to the domain of cortex in which the typical cytoarchitecture was unambiguous. It resulted in smaller volumes of reference, which doubtless influenced the estimated total number of neurons (see Fig. 4, below). However, while the higher variability in neuron number and volume of the analyzed region of interest would not affect neuron somal volume, the lack of a statistically significant difference in neuron number and laminar volumes both within and between groups confirms that the impact of this cytoarchitectural issue in defining the full extent of areas 44 and 45 in these two cases was not large enough to affect the outcome of our study.
Graphs illustrating pyramidal neuron number in patients with autism and controls. Typically developing controls are shown in black and patients with autism in red; children appear as filled in circles and adults as open circles. The asterisks denote two cases in which the cytoarchitectural boundaries of areas 44 and 45 were defined with conservative criteria due to tangential sectioning of the cortical layers (see text for details). Note the lack of differences in estimates of neuron numbers between control cases and patients with autism, and that the two cases in which a more conservative definition of regional boundaries had to be used do not represent statistical outliers
Statistical analysis
We compared eight patients with autism to six control subjects. Our study design consisted of eight pairs with two of the control subjects each matched with two different patients. In view of the matched subject design, we compared each patient with his or her matched control using repeated measures analysis of variance, with the repeated measures being the patient and the control within the pair. In this analysis, we controlled for the average age and the hemisphere that was evaluated for both members of the pair as well as postmortem interval (PMI) and section thickness. For each dependent variable, in addition to the comparison between cases and controls, we also evaluated whether each of the covariates was associated with the difference between cases and controls (e.g., whether there was a differential discrepancy between cases and controls with increasing age and whether there was a differential effect of cases versus controls in the two hemispheres). A p value of 0.05 was used as the criterion for statistical significance. Calculations were performed with SPSS (version 19, IBM SPSS Statistics, New York).
Photography
The photograph of the full brain in Fig. 1 was taken with a Nikon Coolpix P100 and produced with Adobe Photoshop. The hemisphere photograph in Fig. 2 was obtained by scanning a 500-μm thick histologic section of the right hemisphere of the human brain at 1200 dpi (Epson Perfection 2450 Photo Scanner, Epson Electronics America, Long Beach, California). The cortical sections in this figure are virtual slices, which are tiled photomontages of pictures taken with the 10× objective (MBF Bioscience). Photomontages of layer III and V pyramidal neurons in Fig. 3 were taken with the 10× objective and photographs of individual pyramidal neurons were taken with the 40× objective. Adobe Photoshop was used to edit brightness and contrast to optimize images. Graphs in Figs. 4 and 5 were created using GraphPad Prism (GraphPad Prism, version 4; GraphPad Software, San Diego, USA).
Graphs illustrating pyramidal neuron volume in diagnostic groups (a), and across development (b). Typically developing controls are shown in black and patients with autism in red; children appear as filled in circles and adults as open circles. The asterisks denote two cases in which the cytoarchitectural boundaries of areas 44 and 45 were defined with conservative criteria due to tangential sectioning of the cortical layers (see text for details). Note the significantly smaller pyramidal cell volumes in patients with autism and the fact that this effect is more apparent at younger ages
Results
Areas 44 and 45 are dysgranular cortices with a marked columnar cellular organization. The dysgranular cortex is clearly distinct from nearby areas with the presence of many large pyramidal neurons in layer IIIc, a granular layer IV overrun by the both surrounding sublayers IIIc and Va, and large condensed pyramidal neurons in layer Va [2, 8, 75]. The cytoarchitecture in patients with autism and controls was consistent on visual inspection, with the expected cell organization of areas 44 and 45 (Figs. 2, 3). We measured pyramidal neuron number, density, and individual volume, as well as layer volumes in areas 44 and 45, comparing patients with autism and normally developing controls.
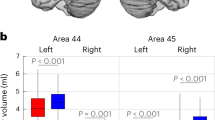
When we used repeated measures analysis of variance, we did not find any statistically significant differences between patients with autism and controls for the volume of layers III, V, and VI, and for the number and density of pyramidal neurons in each layer (Fig. 4; Table 3). We did, however, find statistically significantly smaller pyramidal neuron volume in layers III (18 % smaller than controls; F (1, 5) = 37.869, p = 0.002, Schmitz-Hof CE = 0.077), V (18.5 % smaller than controls; F (1, 5) = 16.258, p = 0.01, Schmitz-Hof CE = 0.098), and VI (22 % smaller than controls; F (1, 5) = 55.737, p = 0.001, Schmitz-Hof CE = 0.105) in patients with autism (Figs. 3, 5a; Table 3). The smaller pyramidal neuron size in patients with autism is visible in Fig. 3c, d compared to the size of pyramidal neurons in controls, shown in Fig. 3a, b.
When we analyzed the extent of interactions with age, we found statistical significance in layers III (p = 0.009) and VI (p = 0.013), but not in layer V (p = 0.067). Examination of the case pairs showed that those with an average age less than or equal to 23 years had a range of differences from 584.43 to 848.77 μm3 and those with an average age above 29 years had differences ranging from 142.03 to 462.16 μm3 in layer III (Fig. 5b). In layer V, the differences in the younger group ranged from 434.36 to 911.93 μm3 and the older group ranged from 112.05 to 493.98 μm3. In layer VI, the differences in the younger group ranged from 642.49 to 822.64 μm3 and from 272.68 to 546.38 μm3 in the older group. Each layer had the same general trend of greater differences between younger patients with autism and controls and smaller differences between older patients and controls; the overlap in differences across ages in layer V is reflected by the p value falling short of significance. This is reflected by the trend lines shown in Fig. 5b, which reveal that young controls have a large pyramidal neuron volume that consistently decreases across their lifespan. Children with autism, however, have smaller pyramidal neuron volumes that are similar to those of adults in both groups (this age effect on pyramidal cell volume was statistically significant only for controls in layer III). The issues related to ambiguous cytoarchitecture in two cases had no impact of neuronal volumes (asterisks, Fig. 5). We found no statistically significant differences for hemisphere, PMI, or section thickness interaction in any of the three layers.
Discussion
Our results reveal a statistically significant reduction in pyramidal neuron size in patients with autism in the inferior frontal cortex, but no difference in pyramidal neuron number or layer volume in layers III, V, and VI, in contrast to recent data on other cortical domains of the frontal lobe [15], suggesting regional specificity in these autism-related differences in neuron numbers. A decreased pyramidal neuron size in autism may have functional repercussion when considered in the context of cortical circuitry. Layer III neurons are involved in corticocortical projections and receive thalamocortical projections [4, 43]. Layer V neurons project cortically and subcortically, primarily to the basal ganglia, brainstem, and spinal cord. Layer VI neurons also project subcortically, predominantly sending feedback projections to the thalamus. Data from studies using diffusion tensor imaging and resting state functional connectivity show that our region of interest is reciprocally connected with the orbital part of the inferior frontal gyrus (area 47), inferior premotor region (area 6), presupplementary motor area, Wernicke’s area (area 22), superior and mid temporal gyri, mid frontal cortex (areas 8 and 9), and the paracingulate gyrus (area 32) [11, 21, 47]. As such, areas 44 and 45 have connections with regions involved in language, movement planning and control, imitation, and emotional perception, suggesting a potential network involved in processing and understanding social communication [33, 34, 48].
Previous findings in the fusiform gyrus and amygdala, both of which send and receive connections with areas 44 and 45, showed reduced neuron number and, in the fusiform gyrus, reduced somatic volume [66, 73]. The alterations found in these areas, which are involved in a face processing system, suggest that neuropathology in one hub of a given network may lead to a disruption in many key areas of that network. If the somatic size of pyramidal neurons is reduced, it is very likely that their functional integrity is compromised. A smaller cell body may also reflect a reduction in dendritic complexity and axonal length [25, 35, 41, 54], which would cause a detrimental effect on connectivity. In this context, Casanova and colleagues investigated minicolumnar size in patients with autism and controls in nine areas of the neocortex, and found the most significant reduction in minicolumnar width in area 44 in patients with autism [10].
These data altogether maintain an established hypothesis of globally reduced connectivity in autism [16]. In support for this hypothesis, functional connectivity is reduced between areas 44 and 45 and temporal language areas [18, 44] as well as with the primary visual cortex [74]. In addition, low-functioning patients with autism have reduced activation of the left inferior frontal gyrus during speech stimulation when compared to controls [52]. Alterations in connectivity of areas 44 and 45 may disrupt language processing, imitation, and joint attention circuits, leading to the language delay that is characteristic of autism.
Whether a lack of fully functioning pyramidal neurons leads to the above impairments, or a lack of salience attribution to social and language cues results in neuronal atrophy or abnormal development remains unclear. In the former case, these smaller neurons may simply be immature, implying that this impairment stems from a defect in the development of pyramidal neurons. This immaturity could be caused by a number of factors, such as an immune disease or infection during pregnancy (e.g., see [19, 26, 57, 63]). In the latter situation, the pyramidal neurons may have been larger early in development, but atrophied soon after due to deficits in functional inputs.
Although we could not separate our sample into developmental subgroups of a sufficiently large size, we qualitatively assessed the distribution of children (defined as less than 21 years of age, [13]) compared to the rest of the sample. There were three children with autism in our study (ages 4, 5, and 8), and two controls (ages 4, a control for both 4- and 5-year-old children with autism, and 8). As shown in Fig. 5, the control children have a larger pyramidal neuron size in each layer, putatively because of the increased connections being developed at this young age. On the other hand, children with autism have consistently smaller neurons, consistent with the notion that children with autism do not develop appropriate connectivity. The fact that young children with autism show pyramidal neuron volumes comparable to those seen in adult controls and adults with autism (Fig. 5b)—in absence of overt pyramidal cell loss that would then indicate that larger neurons were preferentially lost in children with autism—suggests a possible early sign of pyramidal neuron aging, as a reduced pyramidal cell size has been reported in elderly patients [69]. This is an interesting possibility that has not yet been described in autism, and will require confirmation in larger cohorts and detailed quantifications.
Interestingly, in the prefrontal cortex of male children with autism from ages 2 to 16, Courchesne and colleagues recently reported an overall 67 % increase in number of pyramidal neurons as well as increased brain weight in autism [15]. While our areas of interest are located in the prefrontal cortex, our results do not correspond to this striking increase in neuron number. There are several potential reasons that this same increase was not seen. First, Courchesne and colleagues investigated major divisions of the prefrontal cortex, and it is possible that certain areas were more responsible for the increase in neuron number than others. Second, we had a much smaller population of children in our sample, which would certainly bias the outcome of neuron number, given the trajectory of brain growth in autism [14, 36]. Upon closer examination of the young children with autism in the present study, the 4-year-old with autism displayed increased pyramidal neuron number in layers III and V and the 5-year-old had an increase in all three layers, while the 8-year-old had a decrease in the three layers. While these results are not wholly consistent with the prefrontal findings of Courchesne et al. [15], they are reminiscent of the enlargement of the brain in children with autism until approximately age 6 or 7 compared to typically developing controls, which is principally due to an early overgrowth that occurs in the first year [14, 36]. From childhood to preadolescence, the growth of the brain decreases considerably in comparison to growth in age-matched controls [14], and in late adolescence and young adult ages, patients with autism actually have reduced brain size [28, 50]. This trend continues with age, with a premature and accelerated rate of decline in brain size in adults with autism [14]. The lack of difference in layer volumes in our study may be explained by the broad age range or by the combined effects of reduced neuron size with no change in neuron numbers. It is important to keep in mind, however, that the interindividual variability among patients with autism may indeed contribute to some of the discrepancies among neuropathology studies; this highlights the need to study larger samples.
A continuing challenge in autism research is to understand the cellular and molecular causes of the characteristic symptoms that are found throughout the spectrum. Thus far, the pathological basis of neuronal dysfunction in patients with autism remains poorly understood. The significant differences in the size of the pyramidal neurons observed in this study support the hypothesis that patients with autism have reduced connectivity among cortical regions, specifically those involved in social processing [44, 45, 51, 73], and may also provide an explanation for the smaller size of the cortex in patients with autism [14, 28, 50]. Future research will need to determine the cause of the reduction in pyramidal neuron size in areas 44 and 45 in autism. It will also be necessary to perform similar studies in other areas that may be involved in social and language processing to provide greater insight into the potential cellular mechanisms that lead to the impairment of these functions in autism.
References
American Psychiatric Association (2000) ASM-IV-TR: diagnostic and statistical manual of mental disorders text revision. American Psychiatric Association, Washington, DC
Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
Baillargeon R, Scott RM, He Z (2010) False-belief understanding in infants. Trends Cogn Sci 14:110–118
Barbas H (1986) Pattern in the laminar origin of corticocortical connections. J Comp Neurol 252:415–422
Baron-Cohen S, Leslie AM, Frith U (1985) Does the autistic child have a “theory of mind”? Cognition 21:37–46
Bookheimer S (2002) Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25:151–188
Broca P (1861) Remarques sur le siège de la faculté du langage articulé; suivies d’une observation d’aphémie (perte de la parole). Bull Mem Soc Anat Paris 36:330–357
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Johann Ambrosius Barth, Leipzig
Carey DP (1996) ‘Monkey see, monkey do’ cells. Neurophysiology. Curr Biol 6:1087–1088
Casanova MF, El-Baz A, Vanbogaert E, Narahari P, Switala A (2010) A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: possible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathol 20:451–458
Catani M, Jones DK, ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Coleman PD, Romano J, Lapham L, Simon W (1985) Cell counts in cerebral cortex of an autistic patient. J Autism Dev Disord 15:245–255
Council on Child and Adolescent Health, (1988) American Academy of Pediatrics Council on Child and Adolescent Health: age limits of pediatrics. Pediatrics 81:736
Courchesne E, Campbell K, Solso S (2011) Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 1380:138–145
Courchesne E, Mouton PR, Calhoun ME et al (2011) Neuron number and size in prefrontal cortex of children with autism. J Am Med Assoc 306:2001–2010
Courchesne E, Pierce K (2005) Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol 15:225–230
di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (1992) Understanding motor events: a neurophysiological study. Exp Brain Res 91:176–180
Dinstein I, Pierce K, Eyler L et al (2011) Disrupted neural synchronization in toddlers with autism. Neuron 70:1218–1225
Fatemi SH, Earle J, Kanodia R et al (2002) Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol 22:25–33
Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G (2005) Parietal lobe: from action organization to intention understanding. Science 308:662–667
Ford A, McGregor KM, Case K, Crosson B, White KD (2010) Structural connectivity of Broca’s area and medial frontal cortex. Neuroimage 52:1230–1237
Frith U (2001) Mind blindness and the brain in autism. Neuron 32:969–979
Gallese V (2003) The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 36:171–180
Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996) Action recognition in the premotor cortex. Brain 119:593–609
Gilbert CD, Kelly JP (1975) The projections of cells in different layers of the cat’s visual cortex. J Comp Neurol 163:81–105
Goines P, Van de Water J (2010) The immune system’s role in the biology of autism. Curr Opin Neurol 23:111–117
Grezes J, Armony JL, Rowe J, Passingham RE (2003) Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage 18:928–937
Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H (2006) Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 16:1276–1282
Hamilton AF (2009) Goals, intentions and mental states: challenges for theories of autism. J Child Psychol Psychiatry 50:881–892
Hamilton AF, Brindley RM, Frith U (2007) Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia 45:1859–1868
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010) The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50:1313–1319
Happe F, Frith U (1996) The neuropsychology of autism. Brain 119(Pt 4):1377–1400
Hari R, Kujala MV (2009) Brain basis of human social interaction: from concepts to brain imaging. Physiol Rev 89:453–479
Haxby JV, Hoffman EA, Gobbini MI (2000) The distributed human neural system for face perception. Trends Cogn Sci 4:223–233
Hayes TL, Lewis DA (1993) Hemispheric differences in layer III pyramidal neurons of the anterior language area. Arch Neurol 50:501–505
Hazlett HC, Poe MD, Gerig G et al (2011) Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry 68:467–476
Heinsen H, Arzberger T, Schmitz C (2000) Celloidin mounting (embedding without infiltration)—a new, simple and reliable method for producing serial sections of high thickness through complete human brains and its application to stereological and immunohistochemical investigations. J Chem Neuroanat 20:49–59
Heinsen H, Heinsen YL (1991) Serial thick, frozen, gallocyanin stained sections of human central nervous system. J Histotechnol 14:167–173
Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC (2003) The essential role of Broca’s area in imitation. Eur J Neurosci 17:1123–1128
Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999) Cortical mechanisms of human imitation. Science 286:2526–2528
Jacobs B, Driscoll L, Schall M (1997) Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol 386:661–680
Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST (2003) Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron 39:1053–1058
Jones EG (1984) Laminar distribution of cortical efferent cells. In: Peters A, Jones EG (eds) Cellular components of the cerebral cortex. Plenum, New York, pp 521–553
Just MA, Cherkassky VL, Keller TA, Minshew NJ (2004) Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127:1811–1821
Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA (2006) Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain 129:2484–2493
Keller SS, Deppe M, Herbin M, Gilissen E (2012) Variability and asymmetry of the sulcal contours defining Broca’s area homologue in the chimpanzee brain. J Comp Neurol 520:1165–1180
Kelly C, Uddin LQ, Shehzad Z et al (2010) Broca’s region: linking human brain functional connectivity data and non-human primate tracing anatomy studies. Eur J Neurosci 32:383–398
Keuken MC, Hardie A, Dorn BT et al (2011) The role of the left inferior frontal gyrus in social perception: an rTMS study. Brain Res 1383:196–205
Kobayashi C, Glover GH, Temple E (2007) Children’s and adults’ neural bases of verbal and nonverbal ‘theory of mind’. Neuropsychologia 45:1522–1532
Kosaka H, Omori M, Munesue T et al (2010) Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage 50:1357–1363
Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA (2005) Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage 24:810–821
Lai G, Pantazatos SP, Schneider H, Hirsch J (2012) Neural systems for speech and song in autism. Brain 135:961–975
Liakakis G, Nickel J, Seitz RJ (2011) Diversity of the inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behav Brain Res 225:341–347
Lund JS, Lund RD, Hendrickson AE, Bunt AH, Fuchs AF (1975) The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol 164:287–303
Ono M, Kubik S, Abernathey CD (1990) Atlas of the cerebral sulci. Thieme Medical Publishers, New York, pp 54–58
Palmen SJ, van Engeland H, Hof PR, Schmitz C (2004) Neuropathological findings in autism. Brain 127:2572–2583
Patterson PH (2011) Maternal infection and immune involvement in autism. Trends Mol Med 17:389–394
Premack D, Woodruff G (1978) Does the chimpanzee have a ‘theory of mind’? Behav Brain Sci 4:515–526
Rice C (2009) Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ 58:1–20
Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996) Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res 3:131–141
Rizzolatti G, Fogassi L, Gallese V (2001) Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2:661–670
Rogers SJ, Pennington BF (1991) A theoretical approach to the deficits in infantile autism. Dev Psychopathol 3:137–162
Rossignol DA, Frye RE (2012) A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 17:389–401
Santos M, Uppal N, Butti C et al (2011) Von economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res 1380:206–217
Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130:813–831
Schumann CM, Amaral DG (2006) Stereological analysis of amygdala neuron number in autism. J Neurosci 26:7674–7679
Senju A (2011) Spontaneous theory of mind and its absence in autism spectrum disorders. Neuroscientist. doi:10.1177/1073858410397208
Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR (2003) Variability of Broca’s area homologue in african great apes: implications for language evolution. Anat Rec 271:276–285
Terry RD, DeTeresa R, Hansen LA (1987) Neocortical cell counts in normal human adult aging. Ann Neurol 21:530–539
Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A (2005) Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Curr Biol 15:R84–R85
Turella L, Pierno AC, Tubaldi F, Castiello U (2009) Mirror neurons in humans: consisting or confounding evidence? Brain Lang 108:10–21
Tyler LK, Marslen-Wilson WD, Randall B et al (2011) Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain 134:415–431
van Kooten IA, Palmen SJ, von Cappeln P et al (2008) Neurons in the fusiform gyrus are fewer and smaller in autism. Brain 131:987–999
Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA (2005) Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage 25:916–925
von Economo C (1927) L’architecture cellulaire normale de l’écorce cérébrale. Masson, Paris
Wegiel J, Kuchna I, Nowicki K et al (2010) The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol 119:755–770
West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Williams JH (2008) Self-other relations in social development and autism: Multiple roles for mirror neurons and other brain bases. Autism Res 1:73–90
Williams JH, Whiten A, Suddendorf T, Perrett DI (2001) Imitation, mirror neurons and autism. Neurosci Biobehav Rev 25:287–295
Acknowledgments
The authors thank Autism Speaks (the Autism Celloidin Library Project, PRH), the James S. MacDonnell Foundation (PRH), the Seaver Foundation (NU), and the Vachoux Foundation (SJD, MS), Drs J. Wegiel, D. Lightfoot, and J. Pickett, as well as Ms E. Xiu for their generous support. We would also like to acknowledge the Bronx VA Medical Center Brain Bank, Harvard Brain Tissue Resource Center, National Institute of Child Health and Human Development Brain Tissue Bank, New York State Institute for Basic Research in Developmental Disabilities, Oxford Brain Bank, University of Maryland Brain and Tissue Bank, University of Wuerzburg Morphologic Brain Research Unit, and the Autism Tissue Program for providing the materials used in this study. We are especially grateful to the families who donated tissue to make this study possible.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacot-Descombes, S., Uppal, N., Wicinski, B. et al. Decreased pyramidal neuron size in Brodmann areas 44 and 45 in patients with autism. Acta Neuropathol 124, 67–79 (2012). https://doi.org/10.1007/s00401-012-0976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-0976-6