Abstract
Frontotemporal lobar degeneration (FTLD) is generally recognised as a disorder with presenile onset (that is before 65 years of age) with only occasional cases presenting later than this. We set out to determine what proportion of cases of FTLD had late onset of disease and whether such cases of FTLD had distinctive clinical and neuropathological features as compared to cases with presenile onset. Within a combined Manchester and Newcastle autopsy series of 117 cases with pathologically confirmed FTLD (109/117 cases also met Lund Manchester clinical criteria for FTLD), we identified 30 cases (onset age range 65–86 years), comprising 25% of all FTLD cases ascertained in these two centres over a 25-year period. Neuropathologically, the 30 elderly cases presented features of several FTLD histological subgroups [FTLD-TDP (types 1, 2 and 3, 19 cases (63%)], FLTD-tau [MAPT, PiD and CBD, 10 cases (33%)] and FTLD-UPS (1 case), similar in range of phenotypes to that seen in the presenile group, though patients with MAPT, but not PGRN, mutation, or FUS pathology, were notably absent or fewer in the elderly group. Hippocampal sclerosis (HS) was present in 13/30 of the elderly FTLD cases (43%) compared with 14/79 (18%) (P = 0.012) in the presenile FTLD patients. Lobar atrophy present in most of the younger patients was prominent in only 25% of the elderly subjects. Prospective and retrospective psychiatric and medical case note analysis showed that the majority of the elderly FTLD patients, like their younger counterparts, had behavioural features consistent with frontotemporal dementia. FTLD is common amongst elderly persons and all or most of the major clinical and histological subtypes present in younger individuals can be seen in the older group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the literature and in clinical practice, frontotemporal lobar degeneration (FTLD) is generally considered to be a presenile disorder [6, 26]. Indeed, FTLD may be the second most common cause of dementia after Alzheimer’s disease (AD) in the presenile period [11]. Although AD, dementia with Lewy bodies (DLB) and vascular dementia (VaD) are the major causes of dementia in the elderly, cases of FTLD over 70 years of age have been sporadically identified, anecdotally reported or included in reports describing cases of early onset FTLD [3, 16]. Indeed, the Lund and Manchester consensus for FTLD [6] lists an age of 65 years or below as a supportive diagnostic feature of FTLD. Hence, because frontotemporal dementia (FTD) is not usually considered in the differential diagnosis of dementia in patients above 65 years, age alone may mitigate against a diagnosis of FTLD in the elderly patient [6] leading both to misdiagnosis in individual cases and under-representation in epidemiological studies.
The prevalence of FTLD in the elderly is therefore uncertain and often is restricted to clinical identification without neuropathological confirmation. The present study was carried out to determine what proportion of patients within a combined autopsy series of cases of FTLD from the North West and North East regions of United Kingdom had late onset of disease and whether such cases had distinctive clinical and pathological features compared to those of ‘typical’ cases presenting before 65 years of age.
Materials and methods
Over a 25-year period, 87 cases were recruited from the Manchester Brain Bank, 19 with an age of onset over 65 years (see online supplementary Table 1). All were from the North West of England and North Wales and all except one died over the age of 70 years. Similarly, over the same period, 30 cases of FTLD were ascertained from the Newcastle Brain Tissue Resource (NBTR) at the Institute of Human Ageing and Vitality, Newcastle General Hospital, 11 with an age of onset over 65. All were from the North East of England and all died over the age of 70 years (see online supplementary Table 1). Nineteen cases of presenile FTLD were identified during this same period. One hundred and nine (of 117) cases fulfilled clinical diagnostic criteria for FTLD [6, 23]. In six cases a presumptive clinical diagnosis of Alzheimer’s disease/dementia/presenile dementia had been ascribed. Two other cases were victims of road traffic accidents for whom information concerning any pre-existing illness was not known or unavailable. All brains had been obtained with full ethical permission following consent by the next of kin.
Standard macro- and microscopic neuropathology examination was performed on all cases. The brain hemispheres were separated in the fresh state whenever possible, with one hemisphere, the brain stem and cerebellum being fixed in formaldehyde and the other hemisphere being coronally sliced, examined for macropathological features, and then frozen at −80°C. In those instances where asymmetric clinical signs had been present, the whole brain was fixed apart from a few selected blocks that were dissected fresh and frozen. The fixed hemisphere was anatomically sliced and macroscopic features assessed included the absence or presence of infarcts and the pattern and location of brain atrophy.
From the fixed hemisphere a standardised set of representative brain regions was sampled for paraffin embedding and neurohistopathology and sections were cut at a thickness of 6 μm. The following brain areas were reviewed: frontal, temporal, parietal, occipital and motor cortex, deep white matter and internal capsule, hippocampus (CA regions and dentate gyrus, subiculum, presubiculum) entorhinal cortex, basal ganglia (caudate nucleus and putamen) globus pallidus, thalamus, upper and lower midbrain, upper and lower pons and medulla. Spinal cord was only available for examination in eight cases: four cases from Newcastle clinically believed to have FTD, and three cases from Manchester and one from Newcastle considered clinically to have had FTD + MND. Sections to include these regions were stained histologically by H&E, CFV, LFB or Loyez and a silver stain (methenamine-silver, Gallyas, Bielschowsky, or Palmgren). They were also immunostained for phosphorylated tau [mouse monoclonal antibody AT8 (Innogenetics, Ghent, Belgium) 1:750 or rabbit polyclonal tau antibody (Sigma, Poole, UK) 1:200 or tau2 (Sigma, Poole, UK) 1:1,000], amyloid β protein [4G8 mouse monoclonal antibody (Covance Research Products Inc., Dedham, MA) 1:3,000], TDP-43 [rabbit polyclonal antibody (10782-2-AP, Proteintech, Manchester, UK) 1:1,000–1:12,500], FUS protein [rabbit polyclonal antibody HPA-008784 (Sigma, Poole, UK) 1:50–1:200], α-internexin [rabbit polyclonal antibody (Abcam, Cambridge, UK) 1:200], α-synuclein [mouse monoclonal antibody NCL-L-ASYN (Novocastra, Leica Biosystems, Newcastle, UK) 1:40], GFAP [rabbit polyclonal antibody (Sigma, Poole, UK) 1:750]; SMI 31 and 32 (Sternberger-Meyer Immunochemicals, 1: 250); α-internexin (Sigma, Poole, UK) 1:200 or ubiquitin [rabbit polyclonal antibody Z0458 (Dako Cytomation, Ely, UK) 1:750–1:3,000] employing a standard ABC Elite kit (Vector, Burlingame, CA, USA) with DAB as chromagen, and using microwaving in 0.1 M citrate buffer, pH 6.0 for antigen retrieval.
Pathological diagnosis was made according to the algorithm presented by Cairns et al. [7], and FTLD classification made in accordance with Mackenzie et al. [17, 18].
Genetic investigations were performed on DNA extracted either from whole blood or brain tissue by routine methods. DNA was analysed, as described elsewhere, for mutations in MAPT and MAPT haplotype [12, 27], PGRN mutations [4], and APOE genotype [27].
Statistical analysis employing appropriate parametric and non-parametric statistical tests was carried out using SSPS v16.
Results
Demographic details and natural history of disease
The 117 patients with FTLD were divided into two groups according to an age of onset above or below 65 years (Table 1). There were 50 males in the presenile group [mean age at onset (where known) 54.6 ± 8.6 years] and 37 females (mean age 52.2 ± 9.6 years). In the elderly group there were 19 males (mean age 69.2 ± 4.6 years) and 11 females (mean age 72.3 ± 5.5 years). The mean age of onset in the elderly group (70.2 ± 5.1 years) was 16.6 years older than that in their 87 presenile counterparts (53.6 ± 9.1 years), with mean age of death being 14.5 years older (76.4 ± 5.6 vs. 61.9 ± 9.6 years). Overall, disease duration in the elderly FTLD patients was 2.5 years less than that in the presenile group (5.7 ± 2.8 vs. 8.2 ± 3.9 years; P < 0.001), and was also significantly less in older, than younger, males (5.5 ± 2.6 vs. 7.7 ± 4.1 years; P < 0.011) and in older, than younger, females (6.0 ± 3.5 vs. 8.9 ± 3.5 years; P < 0.034). There were no gender differences apparent in either the presenile or elderly group (χ2 = 0.32, P = 0.574).
Clinical presentations
Within the presenile group, 65/87 cases (75%) presented with FTD, seven of these with associated MND. Seven patients (8%) had semantic dementia (SD) and seven (8%) had progressive non-fluent aphasia/anomia/apraxia. Six cases (7%) had no clear diagnosis of their dementia though the clinical picture was not inconsistent with FTLD. Within the elderly group, 23/30 cases (76%) presented with FTD, two of these with associated MND. Two patients (7%) had semantic dementia (SD) and five (17%) had progressive non-fluent aphasia/anomia/apraxia. Hence, all major clinical categories of FTLD were represented in the presenile and elderly groups and there were no clear distinctions in clinical presentation which distinguished the elderly group from their younger counterparts.
Neuropathology
Following review of the histologically and immunohistochemically stained sections it was possible using a recent diagnostic algorithm [7] and present consensus guidelines [17, 18] to characterise and classify most of the 117 cases.
Within the 87 presenile group of cases, there were 31 cases of FTLD-tau (36%), comprising 10 with MAPT mutations (9 with exon 10 +16, 1 with Q336R mutation), 2 with a tauopathy similar to cases with exon 10 +16 mutation but apparently without MAPT mutation being present, 16 with Pick bodies, 2 with CBD or CBD-like histology and 1 with granular globular white matter inclusions). There were 44 cases with FTLD-TDP (50%), 13 with type 1 histology, 15 with type 2 histology and 16 with type 3 histology (5 with PGRN mutations) [7]. There were four cases with FTLD-FUS (5%), classified as atypical FTLD-U, one case of MND alone, four cases with ubiquitinated inclusions that were negative for tau, TDP-43 and FUS (termed FTLD-UPS) and three cases (3%) where no inclusions were detected by immunohistochemistry, and to these a classification of FTLD-ni was ascribed [18].
Within the elderly group, there were ten cases of FTLD-tau (33%), comprising one with MAPT mutation (exon 10 +13 mutation), 1 with a tauopathy similar to cases with exon 10 +16 mutation but apparently without MAPT mutation being present, five with Pick body histology, three with CBD histology. There were 19 cases with FTLD-TDP (63%), 4 with type 1 histology, 4 with type 2 histology and 11 with type 3 histology (2 with PGRN mutations) [7], and 1 case (3%) where only ubiquitinated inclusions were detected by immunohistochemistry, and to this a classification of FTLD-UPS was ascribed [18]. There were no cases with FTLD-FUS. Hence, there were no overall significant differences between the presenile and the elderly group in terms of the frequencies of cases of FTLD-tau (χ2 = 0.05, P = 0.820) or FTLD-TDP (χ2 = 1.46, P = 0.227). However, it was notable that no cases of FTLD-FUS were found in the elderly group.
Cases due to autosomal dominant inheritance associated with mutations in MAPT and PGRN genes (where the results of mutational analysis were known) were more common in the presenile group (15/81 cases, 18.5%) than in the elderly group (3/26 cases, 11.5%), but not significantly so (χ2 = 0.69, P = 0.552). All except one case of MAPT mutation occurred in the presenile group of individuals (10/74 cases, 13.5% vs. 1/24 cases, 4.8% in the elderly group) but this difference was not statistically significant (χ2 = 1.59, P = 0.285). Cases with PGRN mutation were equally common amongst both groups (presenile 5/71 cases, 7.0% vs. elderly 2/23 cases, 8.7%; χ2 = 0.03, P = 1.000).
Where these data were known, there were no significant differences between the presenile and the elderly groups with respect to MAPT H1 haplotype frequency [presenile group (n = 71), H1 = 101/142 alleles 71.1%; elderly group (n = 22), H1 = 30/44 alleles 68.2%, χ2 = 0.71, P = 0.709], or APOE ε2 [presenile group (n = 74), ε2 = 8/148 alleles 5.4%; elderly group (n = 23), ε2 = 1/46 alleles 2.2%, χ2 = 0.83, P = 0.689] or APOE ε4 [presenile group (n = 74), ε4 = 27/148 alleles 18.2%; elderly group (n = 23), ε4 = 7/46 alleles 15.2%, χ2 = 0.0.22, P = 0.825] allele frequencies.
Macroscopic observations on the elderly cases
Average brain weight in the presenile group (1,080 ± 155 g) was slightly less compared to the elderly group (1,126 ± 144 g), but not significantly so (P = 0.196). The majority of the presenile cases showed moderate to severe frontotemporal atrophy and ventricular dilatation, whereas only 12 of the 30 elderly FTLD patients showed severe frontotemporal atrophy and in 8 of the cases severe ventricular dilatation was recorded. There was moderate to severe decrease in neuromelanin pigmentation of the substantia nigra in 10 of the elderly patients, but only so in the locus caeruleus in 5 patients.
Microscopy
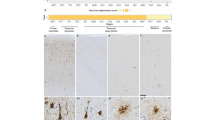
Superficial cortical microvacuolation and neuron loss in the frontal and temporal lobes were identified in all the elderly FTLD cases. Immunohistochemistry showed ubiquitin positivity in all cases. No case was FUS-positive.
In 19 cases, TDP-43 immunoreactive filamentous neurites, neuronal intranuclear (NII) or neuronal cytoplasmic (NCI) inclusions were prominent in the upper and middle cortical layers II and III, as described by us elsewhere [8, 16]. NII were present in five cases, two with known PGRN mutations. NCI were readily seen in the dentate gyrus granular layer. These were classed [7] as 4 with type 1 histology, 4 with type 2 histology and 11 with type 3 histology. Ten cases showed FTLD-tau, comprising AT8-immunopositive Pick bodies (5 cases) or other tau-positive inclusions [neurofibrillary tangles and glial cell inclusions (consistent with MAPT exon 10 +13 or +16 mutations (2 cases) or CBD pathology (3 cases)]. One case displayed ubiquitinated inclusions in the frontal and temporal lobes, but these were not tau, TDP43- or FUS-immunopositive.
Hippocampal sclerosis (HS)
In 13/30 (43%) of the elderly FTLD patients there was a selective and severe loss of pyramidal neurons with gliosis from the hippocampus, involving subfields CA1, subiculum and entorhinal cortex, characteristic of HS, affecting all FTLD subtypes [9 with FTLD-TDP (1 with PGRN mutation), 2 patients with FTLD-tau (PiD) and 1 with FTLD-UPS]. By contrast, HS was present in only 14/79 (18%) of the presenile cases (χ2 = 7.65, P = 0.012), this occurring in nine patients with FTLD-TDP (4 with PGRN mutations), two with FTLD-FUS, one with FTLD-tau (CBD) and one with FTLD-UPS.
Coincidental or age-associated neuropathology
Alzheimer type pathology was present in 13 patients. This was generally in the form of diffuse beta amyloid-positive plaques, with a sparse number of plaques in six cases, a moderate number in six cases and many in a single case. Three patients had Braak stage I, but the likelihood of AD in these patients was low, there being sparse or no plaque scores in these patients [5, 13, 21]. One patient (N29) with FTLD-TDP also had some features of FTLD-tau comprising a moderate to severe degree of neurofibrillary tangles [7] but no evidence of glial cell inclusions, with the phenotype of “tangle only” or neurofibrillary tangle dementia (NTD/FTLD-tau): neuritic or beta amyloid-positive plaques were absent.
Cerebrovascular changes in the elderly FTLD cases
In most patients the major cerebral arteries showed either no, or minimal, atherosclerosis. However, in three patients a bilateral moderate to severe atherosclerosis affected the internal carotid arteries, extending into the middle cerebral arteries. Vascular dementia had been clinically suspected, at least as a component of the illness, in four patients but none of these cases had pathological evidence of infarcts or ischaemic lesions. Small cystic infarcts were recorded in left inferior occipital cortex in one case, and in hypothalamus and upper mid pons, and a focus of ischaemia in the right cerebellar hemisphere in another case, and in a third case an area of microinfarction was present in the hippocampal CA1 region and a small ischaemic area with neuronal loss in the inferior cerebellum.
Discussion
In the present study we have demonstrated that FTLD does indeed occur in subjects with onset over the age of 65 years. When present in these elderly subjects, all the major clinical phenotypes are represented, and as in younger individuals the same features typical of the various histopathological forms of FTLD-TDP and FTLD-tau are seen. Nonetheless, there are some notable differences between the age groups.
First, as might be expected, autosomally dominant inherited gene mutations causing FTLD were more common in the presenile group, though statistically not significantly so given the overall small numbers of mutations present. This was due to all except one of the 12 MAPT mutation carriers being within the younger group whereas PGRN mutations were distributed proportionately between younger and older subgroups.
Secondly, no cases associated with FUS pathology were seen in the older group. The four younger patients with FUS pathology (and atypical FTLD-U) reported here are distinguishable demographically and clinically from the other cases of FTLD. They represent a highly distinctive and unique sub-group characterised by an extremely youthful onset, in keeping with other reports of patients with FUS pathology (and atypical FTLD-U). In one series of 15 patients [24] the mean age at onset was 38 years (range 28–55), in another series of 37 cases, from 12 academic centres [29] it was 41 years, and in a third series of four patients [28], it was 37 years, none of whom were over 50 years at presentation. The present findings would support the argument [28] that onset of FTD before 40 years is a strong predictor of this form of FUS pathology. The patients’ young age is all the more noteworthy in view of the absence of strong family history, again in keeping with other reports [24, 28, 29]. Cases with FTLD associated with other forms of FUS pathology [i.e. those with neuronal intermediate filament inclusion body disease (NIFID) or basophilic inclusion body disease (BIBD)] tend on the whole to have a later onset of age, a different clinical phenotype and different histopathological characteristics. For example, Neumann et al. [25] described five cases of NIFID. The mean age at onset was 40 years though two cases had onset at 56 and 58 years of age. Clinically, the patients showed a typical FTD phenotype, though stereotypical behaviours were not prominent, and unlike the present cases pyramidal motor features were prominent. Striatal atrophy was not excessive and cytoplasmic and nuclear FUS-positive inclusions, similar in appearance to those reported here, were present. These NIFID-associated inclusions were also immunopositive for intermediate filament proteins (i.e. internexin immunoreactive). Munoz et al. [22] reported on seven cases of BIBD. Mean age at onset was 46 years, though here 4/7 cases had onset after 50 years. Clinically, these cases presented with a mixed phenotype with FTD and/or MND being most common presentations, one case presenting as PSP. Stereotypical behaviours were not recorded. Rounded FUS immunopositive inclusions were prominent in the cerebral cortex, but not in hippocampus; NII were absent. Hence, aFTLD-U cases present a distinctive clinical and pathological phenotype which can clearly differentiate them from other forms of FTLD with FUS pathology.
Thirdly, in contrast to the presenile group, HS was significantly more common in the older group. Others have reported that HS is not common in case series with FTLD presenting at an earlier age, although it is being increasingly recognised as a pathological feature of FTLD-TDP [1]. Indeed, in the present study, where HS was observed in younger patients this was nearly always seen in the presence of TDP-43 or FUS pathology. In the elderly group, HS also occurred in conjunction with FTLD-tau (PiD) in two individuals—this association not being seen in the younger group. A recent study has claimed a common variant of PGRN as a risk factor for HS in the elderly [10], though Josephs et al. [15] did not find any difference in the frequency of HS between patients with or without actual PGRN mutations. In the present study, severe HS was seen in 6/7 patients with PGRN mutation.
We [3], and others [14], have shown that HS occurs in some or the majority of FTLD-UPS cases [2], and has been described as a co-existing pathology in cases with FTLD-UPS regardless of subtype [3, 7]. However, Dickson et al. [9] noted HS to be a common pathological feature in dementia of the very old. This was present in 26% of elderly (over 80 years of age) demented subjects though in most individuals defining characteristics of AD or DLB were absent. Dickson used this absence of clear pathological correlation to designate a dementia type (HSD, hippocampal sclerosis dementia) [9]. However, it is not clear to what extent such cases with HS also had associated TDP-43 pathological changes (either in the presence or absence of AD or DLB—conditions in which TDP-43 pathology exists coincidentally in around 25–30% cases).
Patient N29 was of special interest given the additional findings of a moderate to severe degree of neurofibrillary tangles in the absence of neuritic or beta amyloid-positive plaques in whom a diagnosis of “tangle only” or neurofibrillary tangle dementia (NTD/FTLD-tau) was ascribed [7]. A differential diagnosis of dementia pugilistica was considered, but this was excluded given the patient showed no extrapyramidal features and the brain showed no loss of neurones from substantia nigra. However, although no clinical history of single or repeated head injury had been documented, N29 had been formerly actively involved in professional football as player/manager. Consequently, an alternative diagnosis of chronic traumatic encephalopathy (CTE) could also be possible, according to the definition of widespread neurofibrillary tangles in the absence of amyloid plaques, as given by McKee et al. [19]. Moreover, it is interesting that, as in patient N29, 11/12 patients with CTE who were former athletes (i.e. retired boxers, American footballers) involved in contact sports were also reported to show TDP-43 pathological changes [20]. However, the presence of HS in this patient is more in keeping with FTLD than CTE. A definitive diagnosis for this patient therefore remains uncertain.
It is difficult to gauge the true prevalence rate of FTLD in the elderly. In the present series it would seem to be about three times less common than in younger people. Because most of the patients recruited into this study were from the Manchester region and referred to CFU as a specialist referral Centre for early onset dementias, and most of the patients from the Newcastle region were referred through Old Age Psychiatry services, it is highly likely that these prevalence figures are biased towards ascertainment of younger individuals. As based on our Newcastle series, FTLD in the elderly is a comparatively rare disorder and on our consensus estimates is likely to be responsible for between 2 and 3% (maximum 5%) of dementia cases in the elderly. This appears similar to clinical studies in Japan [30]. In the future, these estimates may increase both as a result of increased recognition of FTLD in the elderly, and increased life expectancy and population growth. Prospective studies taking standardised tests into account may offer a way forward. Alternatively, pooling available data in multicenter studies may facilitate a global estimate of the real incidence and prevalence of FTLD in the elderly.
References
Amador-Ortiz C, Lin W-L, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW (2007) Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol 113:245–252
Baborie A, Griffith TG, Jaros E (2008) Frontotemporal dementia in the elderly. Dement Geriatr Cogn Disord 26(S1):P054, 47
Baker M, Mackenzie IRA, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in Progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brun A, Englund E, Gustafson L, Passant U, Mann DMA, Neary D, Snowden JS (1994) Clinical and neuropathological criteria for fronto-temporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 57:416–418
Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VM-Y, Hatanpaa KJ, White CL III, Schneider JA, Tenenholz Grinberg L, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DMA (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown SM, Du Plessis D, Neary D, Snowden JS, Mann DMA (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533
Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (≥80 years of age) humans. Acta Neuropathol 88:212–221
Dickson DW, Baker M, Rademakers R (2010) Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis 7:170–174
Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden M, Pickering-Brown SM, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaf E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd P, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann DM, Lynch T, Heutink P (1998) Association of missense and 5′-splice-site mutation in tau with inherited dementia FTDP-17. Nature 393:702–705
Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097
Josephs KA, Dickson DW (2007) Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Ageing 28:1718–1722
Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve B, Knopman DS, Petersen RC, Davies P, Duara R, Graff-Radford NR, Uittii RJ, Rademakers R, Adamson J, Baker M, Hutton ML, Dickson DW (2007) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 66:142–151
Mackenzie IR, Baborie A, Pickering-Brown SM, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549
Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJM, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DMA (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Mackenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJM, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DMA (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735
McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee H-S, Kubilus CA, Daneshvar DH, Wulff M, Budson AE (2010) TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69:918–929
Mirra SS, Heyman A, McKeel D (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Munoz D, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, Kuroda S, Mackenzie IRA (2009) FUS pathology in basophilic inclusion body disease. Acta Neuropathol 118:617–627
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration—a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA (2009) Mackenzie IR (2009) A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132:2922–2931
Neumann M, Roeber S, Kretzschmar H, Rademakers R, Baker M, Mackenzie IRA (2009) Abundant FUS-immunoreactive pathology in neuronal intermediate filament disease. Acta Neuropathol 118:606–616
Pasquier F, Richard F, Lebert F (2004) Natural history of frontotemporal dementia: comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord 17:253–257
Pickering-Brown SM, Richardson AMT, Snowden JS, McDonagh AM, Burns A, Braude W, Baker M, Liu W-K, Yen S-H, Hardy J, Hutton M, Davies Y, Allsop D, Craufurd D, Neary D, Mann DMA (2002) Inherited frontotemporal dementia in 9 British families associated with intronic mutations in the tau gene. Brain 125:732–751
Seelaar H, Klijnsma KY, de Koning I, van der Lugt A, Chiu WZ, Azmani A, Rozemuller AJM, van Swieten JC (2010) Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol 257:747–753
Urwin H, Josephs KA, Rohrer JD, Mackenzie IR, Neumann M, Authier A, Seelaar H, Van Swieten JC, Brown JM, Johannsen P, Nielsen JE, Holm IE, TheFReJA Consortium, Dickson DW, Rademakers R, Graff-Radford NR, Parisi JE, Petersen RC, Hatanpaa KJ, White Iii CL, Weiner MF, Geser F, Van Deerlin VM, Trojanowski JQ, Miller BL, Seeley WW, van der Zee J, Kumar-Singh S, Engelborghs S, De Deyn PP, Van Broeckhoven C, Bigio EH, Deng HX, Halliday GM, Kril JJ, Munoz DG, Mann DM, Pickering-Brown SM, Doodeman V, Adamson G, Ghazi-Noori S, Fisher EM, Holton JL, Revesz T, Rossor MN, Collinge J, Mead S, Isaacs AM (2010) FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol 120:33–41
Yokota O, Sasaki K, Fujisawa Y, Takahashi J, Terada S, Ishihara T, Nakashima H, Kugo A, Ata T, Ishizu H, Kuroda S (2005) Frequency of early and late-onset dementias in a Japanese memory disorders clinic. Eur J Neurol 12:782–790
Acknowledgments
We thank all staff at the Neuropathology Department, Newcastle General Hospital, and Histopathology Department, Salford Royal Hospitals NHS Foundation Trust for technical assistance, particularly Andrew Brown and Janet Thompson at Newcastle for cutting and staining of the sections and Lynne Ramsay for the TDP43 staining. Dr E. Jaros, Andrew Brown and Lynne Ramsay have been supported by the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Hospitals NHS Foundation Trust. We also acknowledge the support of Alzheimers Research Trust and Alzheimer’s Society through their funding of the Manchester Brain Bank under the Brains for Dementia Research initiative. DMAM and SPB also receive funding from MRC and Wellcome Trust which partially supported this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2010_765_MOESM1_ESM.xls
FTLD pathological classification according to Mackenzie et al. 2010 [18]. FTD = Frontotemporal Dementia, PNFA = progressive non-fluent aphasia, PAX = Progressive apraxia, SD = Semantic dementia, CBD = Corticobasal degeneration, PSP = Progressive Supranuclear Palsy, MND = Motor Neurone Disease, AD = Alzheimer’s Disease, DLB = Dementia with Lewy Bodies, RTA = Road Traffic Accident, FTLD = Frontotemporal lobar degeneration, HS = Hippocampal sclerosis, GGI = Granular Globular Inclusions, Pi = Pick bodies (XLS 44 kb)
Rights and permissions
About this article
Cite this article
Baborie, A., Griffiths, T.D., Jaros, E. et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol 121, 365–371 (2011). https://doi.org/10.1007/s00401-010-0765-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0765-z