Abstract
Baló’s concentric sclerosis (BCS) is considered to be a rare variant of multiple sclerosis and characterized by alternating rings of demyelinated and preserved myelin layers. The mechanism underlying BCS remains to be elucidated. Recently, occurrence of concentric rings of Baló was described in the brainstem of a patient with neuromyelitis optica (NMO). Because selective loss of aquaporin-4 (AQP4) and vasculocentric deposition of complement and immunoglobulins are characteristic in NMO, we aimed to assess AQP4 expression in the concentric demyelinating lesions of BCS patients. We evaluated AQP4 expression relative to expression of another astrocytic marker (glial fibrillary acidic protein), the extent of demyelination, lesion staging and perivascular deposition of complement and immunoglobulin in four cases with BCS, and 30 individuals with other neurological diseases. All cases with BCS demonstrated extensive AQP4 loss in both demyelinated and myelinated layers of all actively demyelinating lesions, with perivascular lymphocytic cuffing of T cells, but no deposition of immunoglobulins or complement around vessels. These findings suggest that AQP4 loss occurs in heterogeneous demyelinating conditions, namely NMO and BCS. Furthermore, acute BCS lesions are characterized by extensive AQP4 loss without vasculocentric deposition of complement or immunoglobulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baló’s concentric sclerosis (BCS), a rare variant of multiple sclerosis (MS), was first described by Baló in 1928 [2]. The initial terminology for this entity was encephalitis periaxialis concentrica, which is based on its early definition of “a disease in the course of which the white matter of the brain is destroyed in concentric layers in a manner that leaves the axis cylinders intact” [2]. This condition is relatively frequently reported in some Asian populations including Filipinos [15], southern Han Chinese [38], and Taiwanese [6]. The clinical course is characterized by an acute onset and steady progression to major disability within a few months. It is pathologically characterized by huge, tumor-like brain lesions showing concentric rings of alternating demyelination and preserved myelin layers [7]. Immunohistochemical analysis of ten cases with BCS suggested that this peculiar concentric pattern formation might be attributable to hypoxia-like tissue preconditioning [34]; however, the primary mechanisms that initiate the lesions remain unknown.
On the other hand, neuromyelitis optica (NMO), another demyelinating disorder, also demonstrating extensive lesions in the spinal cord and optic nerves, is thought to be a variant of MS; however, the recent discovery of a specific immunoglobulin G (IgG) against NMO, designated NMO-IgG [20], suggests that NMO is distinct from MS. This IgG targets the aquaporin-4 (AQP4) water channel protein [19], which is strongly expressed on astrocyte foot processes at the blood–brain barrier (BBB) [12]. Autopsied NMO cases show a loss of AQP4 immunostaining in inflammatory lesions, whereas AQP4 expression is increased in the demyelinating plaques in MS patients [27, 30]. The vasculocentric deposition of complement and immunoglobulins in NMO lesions [23] suggests a humoral immune attack against AQP4 on astrocytes, especially as the NMO-IgG/anti-AQP4 antibody is cytotoxic to astrocytes in vitro and in vivo in the presence of complement [3, 4, 13, 14, 31, 32, 36].
Interestingly, Graber et al. [11] recently reported an occurrence of concentric rings of Baló in the brainstem in an Afro-Caribbean patient with NMO. Because AQP4 status has never been studied in BCS, we performed a systematic immunohistological analysis of AQP4 expression in BCS lesions relative to unaffected white matter areas in the same section, astrocyte marker expression, the extent of demyelination, lesion staging and the perivascular deposition of complement and immunoglobulin in four autopsied cases with BCS. All cases showed loss of AQP4 staining but no perivascular deposition of complement or immunoglobulin in the active concentric lesions, suggesting an occurrence of AQP4-related astrocytopathy also in BCS.
Materials and methods
Autopsy cases of BCS and other neurological disorders
This study was performed on archival autopsy brain materials of six concentric demyelinating lesions from four Filipino cases pathologically diagnosed as BCS. The clinical findings of the patients are summarized in Table 1. The cases consisted of two females and two males, and age at autopsy ranged from 23 to 49 years. Disease durations ranged from 0.1 to 0.6 years (median, 0.4 years). In addition, cases with myasthenia gravis (MG) (n = 2), spastic paraplegia (SPG) type 2 (n = 1), amyotrophic lateral sclerosis (ALS) (n = 6), hippocampal sclerosis with temporal lobe epilepsy (n = 5), muscular dystrophy (n = 1), encephalitis (n = 3), including one with anti-N-methyl-d-aspartate receptor antibody and another with anti-thyroglobulin antibody, spinocerebellar atrophy (SCA) (n = 1), vasculitis (n = 3), cerebral infarction (n = 1), Pick’s disease (n = 1), progressive supranuclear palsy (n = 3) and multiple system atrophy (n = 3) were examined as controls.
Tissue preparation and immunohistochemistry
Autopsy specimens were fixed in 10% buffered formalin and processed into paraffin sections. Sections were routinely stained with hematoxylin and eosin (H&E), Klüver–Barrera (KB) and Bodian or Bielschowsky silver impregnation. The following primary antibodies for immunohistochemistry and staining conditions were used: polyclonal rabbit anti-AQP4 (1:500; Santa Cruz Biotechnology, CA, USA), polyclonal rabbit anti-C3d (1:1000; Dako Cytomation, Glostrup, Denmark), monoclonal mouse anti-C9neo (1:1000; Abcam plc, Cambridge, UK), monoclonal mouse anti-CD68 (1:200; Dako Cytomation, Glostrup, Denmark), monoclonal mouse anti-phosphorylated neurofilament (1:200; Dako Cytomation, Glostrup, Denmark), polyclonal rabbit anti-GFAP (1:1000; Dako Cytomation, Glostrup, Denmark), polyclonal rabbit anti-IgG (1:10000; Dako Cytomation, Glostrup, Denmark), polyclonal rabbit anti-IgM (1:10000; Dako Cytomation, Glostrup, Denmark), monoclonal mouse anti-CD45RO (1:200; Dako Cytomation, Glostrup, Denmark) and monoclonal mouse anti-CD20 (1:200; Dako Cytomation, Glostrup, Denmark). All sections were deparaffinized in xylene and rehydrated in an ethanol gradient. Endogenous peroxidase activity was blocked with 0.3% H2O2/methanol. Antigen retrieval was performed by autoclaving sections in 10 mM citrate buffer pH 6.0 before all antibody incubations except for those against AQP4 and GFAP. The sections were then incubated with primary antibody at 4°C overnight. After rinsing, the sections were subjected to either a streptavidin–biotin complex method or an enhanced indirect immunoperoxidase method using Envision (Dako Cytomation, Glostrup, Denmark). Immunoreactivity was detected using 3,3′-diaminobenzidine and sections were counterstained with hematoxylin. Immunohistochemistry for activated complement, immunoglobulins, T cell and B cell markers was performed on randomly selected lesions.
Staging of demyelinating lesions
We classified demyelinating lesions into the following three stages: actively demyelinating lesions, chronic active lesions and chronic inactive lesions based on the density of macrophages phagocytizing myelin debris [16]. Briefly, actively demyelinating lesions were active destructive lesions densely and diffusely infiltrated with macrophages phagocytizing myelin debris, as identified by Luxol fast blue staining. Chronic active lesions were those showing hypercellularity of macrophages restricted to the periphery of the lesions. Chronic inactive lesions were those showing no increase in the numbers of macrophages throughout the lesions. According to the protocol, the six concentric lesions studied were all staged to actively demyelinating lesions.
Comparison of AQP4 expression with myelin loss and astrogliosis
For each lesion, we compared the level of AQP4 expression with the spatial distribution of myelin loss. AQP4 expression levels in region-matched unaffected areas (i.e. gray vs. white mater) in the same section were used as an internal control. To exclude seeming AQP4 down-regulation due to the total loss of astrocytes in such lesions as cavity formation and those totally replaced by macrophages, and to strictly evaluate the AQP4 expression status in the preserved astrocytes in and around the lesions, we confirmed the existence of astrocytes by GFAP staining of neighboring sections for all lesions.
Results
Immunohistochemical findings in control brains
AQP4 expression in pathologically normal brains
AQP4 in normal cerebral tissues from an MG case was diffusely expressed in the cortex, staining the fine processes of the cortical astrocytes (Fig. 1a). Astrocytic perivascular foot processes were more strongly stained with AQP4 than the background neuropil in the cortical gray matter (Fig. 1b, arrows). The glial limiting membranes and subependymal astrocytes also strongly expressed AQP4 (Fig. 1a). There was less AQP4 staining in the white matter than in the cortex, with staining primarily in the perivascular foot processes (Fig. 1c, arrows). In contrast, GFAP immunoreactivity was preferentially observed in the cerebral white matter (Fig. 1d, e). In the cortex, except for the strong staining of the glial limiting membranes (Fig. 1d, arrow), only a few astrocytes were immunopositive for GFAP, with less staining in the perivascular foot processes (Fig. 1f, arrows).
AQP4 and GFAP expression in normal control brains. A case of MG. AQP4 immunoreactivity is most intense in the glial limiting membrane (arrow) and the subependymal astrocytes (arrowhead) in the cerebrum. AQP4 is diffusely expressed in the cortex with neuropil staining, while the white matter shows only weak staining (a). Higher magnification of the cortex shows strong AQP4 expression in the perivascular foot processes of the cortical astrocytes (arrows) (b). Higher magnification of the white matter demonstrates an AQP4 staining mainly in the perivascular foot processes of the astrocytes (arrows) (c). GFAP immunoreactivity is stronger in the white matter than in the cortex. The glial limiting membrane also stains for GFAP (arrow) (d). Higher magnification of the white matter shows strong GFAP immunoreactivity (e). Higher magnification of the cortex shows only scattered GFAP immunoreactive cortical astrocytes and faint staining in the vascular foot processes of astrocytes (arrows) (f). In the cerebellar cortex, GFAP stains Bergmann glia with radial cytoplasmic processes (g). In the cerebellar cortex, AQP4 immunoreactivity is observed in Bergmann glia with radial cytoplasmic processes (h). AQP4 (a–c, h) and GFAP (d–g) immunohistochemistry. Scale bar 300 μm (a, d), 50 μm (b, c, e–h), AQP4 aquaporin-4, GFAP glial fibrillary acidic protein, MG myasthenia gravis
In the cerebellar cortex, both GFAP and AQP4 were expressed in Bergmann glia with radial cytoplasmic processes (Fig. 1g, h), although AQP4 immunoreactivity was generally weaker than that for GFAP. The glial limiting membranes of the cerebellum also strongly expressed GFAP as well as AQP4. In the cerebellar white matter, AQP4 immunoreactivity was mainly observed in the perivascular foot processes, as seen in the cerebral white matter (data not shown).
AQP4 expression in areas of astrogliosis
The normal expression pattern for AQP4 expression was different from that for GFAP as described. However, areas of astrogliosis were generally immunopositive for AQP4 as well as GFAP, in both the gray matter and white matter, regardless of disease types (Fig. 2). For example, hypertrophic gemistocytes in cases of limbic encephalitis (Fig. 2a, b), cerebral infarction (Fig. 2c, d) and SPG type 2 (Fig. 2e, f) showed surface staining for AQP4 in the cytoplasm and processes. Fibrillary gliosis or gliotic scars also showed diffuse AQP4 staining along the glial fibers in cases of SPG type 2 (Fig. 2g, h) and hippocampal sclerosis (data not shown).
AQP4, immunoglobulin and complement expression patterns in cases with other neurological diseases. (a–h) AQP4 expression in astrogliosis. GFAP-positive gemistocytes (a, c, e) show membranous staining for AQP4 (b, d, f) in cases of limbic encephalitis (a, b), cerebral infarction (c, d) and SPG type 2 (e, f). Asterisks in c and d indicate necrotic areas. Fibrillary gliosis seen in the corpus callosum of the same SPG type 2 case is positive for both GFAP (g) and AQP4 (h). i–n Deposition of immunoglobulins and activated complement (C3d and C9neo) in cases with other neurological diseases. Serial sections distant from focal lesions in the pons from a case with SCA. C3d staining is detected in the perivascular areas (i). IgM staining shows a similar pattern to C3d staining (j). (k–n) Serial sections of the cerebral tissues from a case with cerebral infarction. Numerous macrophages filled with C3d-positive granules in the lesions (inset macrophages) (k). C9neo staining shows a similar pattern to C3d staining (inset macrophages) (l). IgM staining shows a similar pattern to activated complement staining (inset macrophages) (m). IgG staining shows the similar pattern as IgM staining. In addition, staining outlining the cytoplasm of hypertrophic astrocytes was noted (inset arrowheads) (n). GFAP (a, c, e, g), AQP4 (b, d, f, h), C3d (i, k), IgM (j, m), C9neo (l) and IgG (n) immunohistochemistry. Scale bar 100 μm (i, j), 50 μm (k–n), 25 μm (a–h), AQP4 aquaporin-4, GFAP glial fibrillary acidic protein, SPG spastic paraplegia, SCA spinocerebellar atrophy
Deposition of immunoglobulins and activated complement
Immunohistochemistry for immunoglobulins and activated complement in cerebral tissues from cases with MG, ALS, SCA, vasculitis, limbic encephalitis and cerebral infarction demonstrated weak, diffuse IgG immunoreactivity in the neuronal soma, neuropil, oligodendrocytes, astrocytes, glial limiting membranes and ependymal epithelium, but not in the white matter (data not shown). IgM, C3d and C9neo immunoreactivities were only focally detected in the control cases, and whenever present they were generally confined to blood vessel walls and perivascular regions. Activated complement was not usually co-localized with immunoglobulins; however, in 4 of the 17 cases with non-inflammatory diseases (one each with ALS, SCA, progressive supranuclear palsy and multiple system atrophy) and two of the six cases with inflammatory disorders (anti-N-methyl-d-aspartate receptor antibody-seropositive limbic encephalitis and anti-thyroglobulin antibody-seropositive encephalitis), focal perivascular staining for both C3d and IgM was occasionally observed (Fig. 2i, j). In the lesions of patients with ischemic infarction, foamy macrophages were commonly stained for C3d, C9neo, IgM and IgG. In addition, surface staining of reactive astrocytes was seen with IgG immunostaining, probably due to diffusion of the serum in the affected brain tissue (Fig. 2k–n).
Immunohistochemical findings in BCS
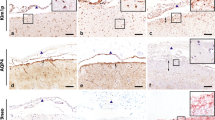
All cases with BCS showed concentric rings of alternating demyelination and preserved myelin layers in the cerebral white matter (Fig. 3a, d). The lesion center was entirely covered with GFAP immunostaining. In the centers of all six actively demyelinating lesions, AQP4 staining was markedly diminished, despite the strong GFAP immunoreactivity (Table 2, Fig. 3c, f, g–l) in both gemistocytic astrocytes (Fig. 3h, i) and astrocytic vascular foot processes (Fig. 3k, l) compared with the unaffected white matter regions with preserved myelin staining (Fig. 3j). The myelin staining negative, peripheral layers of the lesions showed marked decreases of both GFAP and AQP4 staining. High-power field inspection revealed that these areas were almost totally replaced by foamy macrophages (Fig. 3m), with only a small number of highly degenerated GFAP-positive astrocytic processes (Fig. 3n) and axon fragments positive for silver staining (Fig. 3o) and phosphorylated neurofilaments (Fig. 3p). Despite the existence of a few GFAP-positive structures, these areas were totally devoid of AQP4 staining (Fig. 3q). In all lesions, perivascular cuffing with lymphocytes was observed (Fig. 3r). There was dense infiltration of foamy macrophages including myelin debris in the demyelinating layers (Fig. 3s). The perivascular infiltrates predominantly consisted of T cells, while vasculocentric deposition of immunoglobulins (IgG and IgM) or activated complement (C3d and C9neo) was never observed in any of the lesions examined (Table 2; Fig. 3t–v).
Representative AQP4 expression pattern in concentric or lamellar demyelinating lesions. Serial sections of the cerebral tissue with actively demyelinating lesions in the white matter from case Baló-1 (a–c) and case Baló-2 (d–s). The cerebral white matter reveals concentric or lamellar demyelinated lesions (a, d). GFAP is expressed in the lesion centre, but is largely diminished in the lamellar necrotic foci at the lesion edge (arrows) (a, b, d, e). AQP4 immunoreactivity is largely lost in the lesion centre with lamellar myelin-staining patterns (c, f). Numerous reactive, hypertrophic astrocytes are seen in both the demyelinating and preserved myelin layers (g). Astrocytes in (g) strongly express GFAP (h) but lack surface staining for AQP4 (i). In the unaffected white matter with preserved myelin staining and no inflammatory infiltration in the same section (the area indicated by arrowhead in d), non-reactive astrocytes show AQP4 staining on the cell surface and their processes (j) and AQP4 expression is also preserved in the perivascular astrocytic foot processes (k). In the lesions, however, the perivascular AQP4 staining also disappears (l). High-power field views of GFAP-negative necrotic foci at the lesion edge (indicated by arrows in a, b, d, e) reveal dense infiltration of foamy macrophages (m), a small number of highly degenerative, remaining astrocytes (n), axons (arrowheads in o, p) and foamy spheroids (arrows in p). AQP4 immunoreactivity is totally lost in the necrotic areas (q). Perivascular accumulation of lymphocytes is noted all over the lesion (r). Dense infiltration of foamy macrophages phagocytosing myelin debris in the demyelinating layer (s). Perivascular lymphocytes are immunopositive for the T cell marker CD45RO (t), but negative for the B cell marker CD20 (u). IgG, IgM, C3 and C9neo labeling is detected in some glial cells, but not in the perivascular areas (v). Klüver–Barrera staining (a, d, s), hematoxylin and eosin staining (g, m, r), GFAP (b, e, h, n) AQP4 (c, f, i–l, q), Bielschowsky’s silver staining (o), phosphorylated neurofilament (p), CD45RO (t), CD20 (u) and IgG, IgM, C3 and C9neo (v) immunohistochemistry. Scale bar 4 mm (a–f), 100 μm (v), 50 μm (k, l, t, u), 25 μm (g–j, m–s). AQP4 aquaporin-4, GFAP glial fibrillary acidic protein
Discussion
We performed an immunohistopathological study on AQP4 expression in autopsy cases of BCS. Surprisingly, all cases with BCS uniformly demonstrated extensive AQP4 loss in all actively demyelinating lesions with perivascular lymphocytic cuffing, but no deposition of immunoglobulins or complement around the blood vessels. Our study indicates that AQP4 loss could occur not only in NMO but also in BCS lesions.
We found a similar AQP4 expression pattern in normal brain tissues to what has been previously reported [27, 30], although we used a different anti-AQP4 antibody to the ones used in those studies. For example, normal cortical astrocytes were diffusely immunostained for AQP4 and weakly immunostained for GFAP, whereas white matter astrocytes showed a reverse pattern. AQP4 is strongly expressed in the glial limiting membranes, and subependymal and perivascular astrocytes. In control diseased brains, both reactive, gemistocytic astrocytes and areas of chronic fibrillary gliosis showed high levels of AQP4 expression regardless of the cause, as previously reported [1, 18, 27, 30]; however, since all the BCS lesions examined in this study were acute lesions, we did not observe chronic fibrillary gliosis and resultant upregulation of AQP4. On the other hand, demyelinating lesions with AQP4 loss generally showed numerous GFAP-immunopositive, gemistocytic astrocytes. In addition, AQP4 expression was lost from GFAP-positive perivascular foot processes. Although some of the myelin-negative foci at the periphery of the concentric lesions showed a decrease in both GFAP and AQP-4 staining, these areas were proven to be necrotic foci with only a small amount of debris from astrocytic processes and axons. Therefore, acute BCS lesions are characterized by AQP4 loss in GFAP-expressing astrocytes and their vascular foot processes, distinct from the staining pattern in NMO in which astrocytes reportedly lose both GFAP and AQP4 staining [27].
This is the first report to show AQP4 loss in BCS cases, not only in the layers showing demyelination, but also in the layers with preserved myelin. All actively demyelinating lesions in BCS showed the same pattern of AQP4 loss, but other acute inflammatory conditions such as limbic encephalitis did not show such AQP4 loss in lesions, suggesting that AQP4 loss is an inherent component of this acute disease. We are currently examining AQP4 status in NMO and MS cases in comparison with that in BCS cases.
All autopsied materials were archival ones taken long before the discovery of NMO-IgG/anti-AQP4 antibody, and the anti-AQP4 antibody statuses of the present BCS cases are unknown. However, because the vasculocentric deposition of complement and immunoglobulins was not confirmed in any of the BCS lesions, an autoantibody and complement-mediated mechanism, which is considered to be unique to NMO, may not be operative in BCS. In future, it will be necessary to measure levels of anti-AQP4 antibody in a large clinical series of BCS patients.
The lesions in BCS are classified as type 3 lesions as described by Lucchinetti et al. [22], and the disease is considered to be an oligodendrocytopathy. However, we found AQP4 down-regulation in both demyelinated and myelinated layers, suggesting that astroglial damage occurs more widely than oligodendroglial damage. Hypoxia-like tissue injury may contribute to Baló’s lesions [24, 34], which can show restricted diffusion on diffusion-weighted MRI sequences, as happens in acute stroke [39]. Because AQP4 is down-regulated in hypoxic conditions and in the ischemic core at the acute stage [9, 10, 17, 26], vessel obliteration or mitochondrial impairment [24] associated with heavy lymphocytic inflammation may lead to tissue hypoxia and AQP4 down-modulation in BCS.
It has recently been reported that reactive astrocytes that form perivascular scars act as barriers to leukocytes and that conditioned ablation of reactive astrocytes strengthens inflammation [37]. Because AQP4 deletion impairs glial scar formation [35], down-regulation of AQP4 in BCS may enhance inflammatory changes through interruption of perivascular glial scar formation. On the other hand, AQP4 knockout mice show reduced cytotoxic edema following tissue ischemia and hypoxia [25], suggesting that AQP4 down-regulation is protective. Similarly, experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein peptide is attenuated in AQP4 knockout mice [21]. These observations suggest that AQP4 down-modulation could be neuroprotective in hypoxia-induced cytotoxic edema as well as in inflammatory demyelinating lesions, which may also be applicable to BCS. On the other hand, it has been reported that AQP4 inhibition causes exacerbation of vasogenic edema [29]. The formation of tumor-like, highly edematous BCS lesions thus might mainly result from vasogenic edema.
In MS, AQP4 loss in actively demyelinating lesions has so far not been reported; Misu et al. [27] found no loss or exaggerated expression of AQP4 in MS plaques, while Roemer et al. [30] detected AQP4 loss in the chronic inactive lesions, suggesting that stage-dependent loss of AQP4 may occur in some MS lesions. Therefore, extensive AQP4 loss in the acute lesions without vasculocentric deposition of complement or immunoglobulin is considered to be characteristic of BCS.
There are several lines of experimental evidence that anti-AQP4 antibody-independent impairment of astrocytes causes AQP4 loss followed by demyelination. Sharma et al. [33] reported lipopolysaccharide-induced demyelinating lesions in rats showed loss of AQP4 and retraction of astrocytic vascular foot processes. Likewise, Wolburg-Buchholz et al. [40] demonstrated that ultrastructural distribution of AQP4 is altered in the astrocytic foot processes followed by destruction of the BBB in a murine experimental autoimmune encephalomyelitis model immunized with proteolipid protein peptide. Indeed, the astrocytes in the active lesions in our BCS cases showed extremely hypertrophic, abnormal reacting morphology suggestive of considerable functional impairment including loss of AQP4 expression. The mechanism underlying such anti-AQP4 antibody-independent AQP4 loss is currently unknown. However, phosphorylation-related internalization of AQP4 resulting from a variety of stimuli might initiate this process [5, 8, 28].
Future studies on astrocytopathy as well as the dynamic plasticity of astrocytes may shed light on the mechanisms underlying the alternating myelinated and demyelinated lesions observed in BCS.
References
Aoki K, Uchihara T, Tsuchiya K, Nakamura A, Ikeda K, Wakayama Y (2003) Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol 106:121–124
Baló J (1928) Encephalitis periaxialis concentrica. Arch Neurol 19:242–263
Bennett JL, Lam C, Kalluri SR et al (2009) Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 66:617–629
Bradl M, Misu T, Takahashi T et al (2009) Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol 66:630–643
Carmosino M, Procino G, Tamma G, Mannucci R, Svelto M, Valenti G (2007) Trafficking and phosphorylation dynamics of AQP4 in histamine-treated human gastric cells. Biol Cell 99:25–36
Chen CJ, Chu NS, Lu CS, Sung CY (1999) Serial magnetic resonance imaging in patients with Baló’s concentric sclerosis: natural history of lesion development. Ann Neurol 46:651–656
Courville CB (1970) Concentric sclerosis. In: Vinken PJ, Bruyn GW (eds) Multiple sclerosis and other demyelinating diseases. North-Holland, Amsterdam, pp 437–451
Fenton RA, Moeller HB, Zelenina M, Snaebjornsson MT, Holen T, MacAulay N (2010) Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol Life Sci 67:829–840
Frydenlund DS, Bhardwaj A, Otsuka T et al (2006) Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci USA 103:13532–13536
Fujita Y, Yamamoto N, Sobue K et al (2003) Effect of mild hypothermia on the expression of aquaporin family in cultured rat astrocytes under hypoxic condition. Neurosci Res 47:437–444
Graber JJ, Kister I, Geyer H, Khaund M, Herbert J (2010) Neuromyelitis optica and concentric rings of Baló in the brainstem. Arch Neurol 66:274–275
Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P (1994) Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA 91:13052–13056
Kinoshita M, Nakatsuji Y, Kimura T et al (2009) Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun 386:623–627
Kinoshita M, Nakatsuji Y, Moriya M et al (2009) Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. Neuroreport 20:508–512
Kuroiwa Y (1985) Neuromyelitis optica (Devic’s disease, Devic’s syndrome). In: Koetsier JC (ed) Handbook of clinical neurology. Demyelinating disease, vol 47. Elsevier, Amsterdam, pp 397–408
Lassmann H, Raine CS, Antel J, Prineas JW (1998) Immunopathology of multiple sclerosis: report on an international meeting held at the institute of neurology of the University of Vienna. J Neuroimmunol 86:213–217
Lee M, Lee SJ, Choi HJ et al (2008) Regulation of AQP4 protein expression in rat brain astrocytes: role of P2X7 receptor activation. Brain Res 1195:1–11
Lee TS, Eid T, Mane S et al (2004) Aquaporin-4 is increased in the sclerotic hippocampus in human temporal lobe epilepsy. Acta Neuropathol 108:493–502
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202:473–477
Lennon VA, Wingerchuk DM, Kryzer TJ et al (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112
Li L, Zhang H, Verkman AS (2009) Greatly attenuated experimental autoimmune encephalomyelitis in aquaporin-4 knockout mice. BMC Neurosci 10:94
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Lucchinetti CF, Mandler RN, McGavern D et al (2002) A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125:1450–1461
Mahad D, Ziabreva I, Lassmann H, Turnbull D (2008) Mitochondrial defects in acute multiple sclerosis lesions. Brain 131:1722–1735
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW et al (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Meng S, Qiao M, Lin L, Del Bigio MR, Tomanek B, Tuor UI (2004) Correspondence of AQP4 expression and hypoxic-ischaemic brain edema monitored by magnetic resonance imaging in the immature and juvenile rat. Eur J Neurosci 19:2261–2269
Misu T, Fujihara K, Kakita A et al (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130:1224–1234
Moeller HB, Fenton RA, Zeuthen T, Macaulay N (2009) Vasopressin-dependent short-term regulation of aquaporin 4 expressed in Xenopus oocytes. Neuroscience 164:1674–1684
Nag S, Manias JL, Stewart DJ (2009) Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol 118:197–217
Roemer SF, Parisi JE, Lennon VA et al (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130:1194–1205
Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC (2010) Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133:349–361
Sabater L, Giralt A, Boronat A et al (2009) Cytotoxic effect of neuromyelitis optica antibody (NMO-IgG) to astrocytes: an in vitro study. J Neuroimmunol 215:31–35
Sharma R, Fischer MT, Bauer J et al (2010) Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol 120:223–236
Stadelmann C, Ludwin S, Tabira T et al (2005) Tissue preconditioning may explain concentric lesions in Baló’s type of multiple sclerosis. Brain 128:979–987
Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC (2006) Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta 1758:1085–1093
Vincent T, Saikali P, Cayrol R et al (2008) Functional consequences of neuromyelitis optica–IgG astrocyte interactions on blood–brain barrier permeability and granulocyte recruitment. J Immunol 181:5730–5737
Voskuhl RR, Peterson RS, Song B et al (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522
Wang C, Zhang KN, Wu XM et al (2008) Baló’s disease showing benign clinical course and co-existence with multiple sclerosis-like lesions in Chinese. Mult Scler 14:418–424
Wiendl H, Weissert T, Herrlinger U, Krapf H, Küker W (2005) Diffusion abnormality in Baló’s concentric sclerosis: clues for the pathogenesis. Eur Neurol 53:42–44
Wolburg-Buchholz K, Mack AF, Steiner E, Pfeiffer F, Engelhardt B, Wolburg H (2009) Loss of astrocyte polarity marks blood–brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol 118:219–233
Acknowledgments
We thank Ms. Sachiko Nagae and Ms. Kimiko Sato, Department of Neuropathology, Kyushu University, for their excellent technical assistance and Dr. Takekazu Ohi, Department of Neurology, Kurashiki Central Hospital for providing materials. This work was supported in part by Grants from the Research Committees of Neuroimmunological Diseases, the Ministry of Health, Labor and Welfare, Japan (JK) and from the Ministry of Education, Culture, Sports, Science and Technology (JK), Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuoka, T., Suzuki, S.O., Iwaki, T. et al. Aquaporin-4 astrocytopathy in Baló’s disease. Acta Neuropathol 120, 651–660 (2010). https://doi.org/10.1007/s00401-010-0733-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0733-7