Abstract
Neuromyelitis optica (NMO) is an autoimmune inflammatory disorder of the CNS with a predilection for the optic nerves and spinal cord, often with longitudinally extensive transverse myelitis (LETM) on MRI. The discovery of aquaporin-4 (AQP4)-immunoglobulin G (IgG), an antibody against the astrocyte water channel in the CNS, clearly identified NMO as a disease separate from MS. The high specificity of AQP4-IgG has permitted recognition of a wider spectrum of clinical and radiologic features related to NMO. Other sites of CNS involvement not restricted to the optic nerves or spinal cord have been described in AQP4-IgG-seropositive patients such as the diencephalon, brainstem, and brain hemispheric white matter. In anti-AQP4-IgG-seropositive patients, partial clinical (e.g., only myelitis) and MRI findings have been recognized, while conversely, NMO-like MRI findings and clinical features can occur in anti-AQP4-IgG-seronegative patients, sometimes with anti-MOG antibodies.
The term NMO spectrum disorder (NMOSD) has been adopted to reflect the clinical, serological, and radiological diversity. Clinical features alone may be insufficient to diagnose NMOSD; cerebrospinal fluid (CSF) analysis and radiological techniques, in particular MRI, are required to exclude other disorders and reveal the characteristic optic nerve, spinal cord, and cerebral findings. The value of MRI in the diagnostic process is particularly relevant in patients who are anti-AQP4-IgG negative or when serologic testing is unavailable. This chapter gives special consideration to the value of clinical neuroradiology in the diagnosis of NMOSD and in the distinction from MS (with short segment lesions rather than LETM) and other immune-mediated inflammatory demyelinating diseases.

This publication is endorsed by: European Society of Neuroradiology (www.esnr.org).
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Neuromyelitis optica spectrum disorder (NMOSD)
- Aquaporin-4-immunoglobulin G antibodies (anti-AQP4-IgG antibodies)
- Magnetic resonance imaging (MRI), Longitudinally extensive transverse myelitis (LETM), Optic neuritis (ON)
- Myelin oligodendrocyte glycoprotein immunoglobulin G antibodies (anti-MOG-IgG antibodies)
Definition of Entity: Neuromyelitis Optica Spectrum Disorder (NMOSD)
Neuromyelitis optica (NMO), also known as Devic’s disease, is a rare, severely disabling, autoantibody-mediated chronic inflammatory disease affecting the whole CNS. For many years it was considered to be a subtype of multiple sclerosis (MS) but nowadays is accepted as an independent disease. NMO is characterized by severe attacks of optic neuritis (ON), longitudinally extensive transverse myelitis (LETM), and less frequently intractable nausea, hiccups, and vomiting due to involvement of the area postrema. In 2004, the discovery of highly specific serum immunoglobulin-G (IgG) antibodies directed against the aquaporin-4 (AQP4) water channel enabled the recognition of a more diverse clinical spectrum of disease manifestations. Antibody testing was incorporated into the diagnostic criteria of NMO for the first time in 2006 (Wingerchuk et al. 2006).
The identification of anti-AQP4 antibodies beyond the classic presentation of NMO indicated a broader clinical phenotype of this disorder, the so-called NMO spectrum disorder (NMOSD); it encompasses anti-AQP4-IgG seropositive patients with limited or inaugural forms of NMO and with specific brain abnormalities (Kim et al. 2015). On the other hand, AQP4-seronegative NMOSD patients with the same characteristic symptoms may have serum antibodies against myelin oligodendrocyte glycoprotein (MOG), also referred to as MOG-Ab-associated disease.
MRI has an increasingly important role in differentiating NMOSD from other neuroinflammatory disorders with similar symptoms, particularly MS, but also rheumatological diseases that may occur concomitantly with NMOSD, such as systemic lupus erythematosus and Sjögren syndrome. The differentiation between these disease entities is clinically crucial as treatments are distinct. A summary of NMOSD characteristics are described in Fig. 1.
Pathophysiology
The pathogenic role of astrocytes as the initial target of the autoimmune attack in NMOSD has been increasingly recognized. The highly CNS-specific AQP4-IgG antibody is predominantly expressed on perivascular astrocytic processes. AQP4 is the main channel regulating water homeostasis within the CNS and is highly concentrated in the foot processes that make contact with capillary endothelia forming the blood-brain barrier and in the ependymal cells at brain-CSF interfaces. AQP4 expression is reduced in NMOSD. The discovery of autoantibodies, such as AQP4-IgG, followed by MOG-IgG, and likely others more to come (e.g., against glial fibrillary acidic protein [GFAP]), has broadened the differential diagnosis of neuroinflammatory diseases. Today, in the majority of patients with NMOSD, serum autoantibodies against astrocytic AQP4 water channels (or MOG) are detectable. Various anteceding or coexisting infections, vaccinations, or systemic autoimmune diseases have been linked to NMOSD, but the cause of the disease remains unknown.
Histopathologically, lesions in AQP4-IgG positive NMOSD are characterized by massive astrocyte destruction with deposition of immunoglobulins, activated complement, and granulocyte infiltration. During the acute phase, MRI of affected optic nerves and spinal cord may reveal swelling and contrast enhancement caused by blood-brain barrier breakdown. These features are probably triggered by damage to astrocytic end-feet at the glia limitans of the blood-brain barrier. Subsequently to the acute inflammatory phase, cystic changes with severe tissue destruction and Wallerian degeneration may occur.
Clinical Features and Epidemiology
The classical features of NMO include bilateral optic neuritis and myelitis occurring either simultaneously or in short succession. Patients typically suffer from recurrent attacks of mostly unilateral optic neuritis and/or myelitis, which usually occur sequentially rather than simultaneously. Ocular pain with loss of vision, severe (symmetric) paraplegia, sensory loss below the cord lesion, and bladder dysfunction are typical clinical features. In rarer cases, brainstem and brain involvement, e.g., area postrema or diencephalic syndromes, can occur, resulting in nausea, hiccups, or acute neurogenic respiratory failure. Patients also suffer from other burdensome symptoms like headache, pain, depression, fatigue, and sleep disorders (Table 1).
NMOSD usually follows a relapsing course in approximately 90% of cases, often without complete recovery, causing cumulative irreversible deficits, and often rapid accumulation of disability. Within 5 years from disease onset, more than 50% of patients are blind in one or both eyes or require ambulatory support. Disease onset ranges between 4 and 88 years (mean age of onset 39 years), with a female to male ratio in the AQP4-IgG-group of up to 9:1. While 90% of patients relapse within 5 years after the index event, some may have a much longer disease-free period. NMO prevalence is estimated between 0.5 and 4.4/100,000 and is more frequent in Southeast Asia and Latin America.
In 20–30% of NMOSD patients, depending on the assay used, anti-AQP4 serum antibodies cannot not be detected. For clinical purposes, cell-based assays are recommended due to their superior sensitivity and specificity over other assays.
Opticospinal MS and NMOSD in Asia
Asian MS tends to involve the optic nerve and the spinal cord more frequently, with less brain parenchymal involvement, clinically resembling relapsing-remitting NMOSD in Caucasians. Furthermore, Asian MS patients have a higher mean age at diagnosis, higher relapse rates, and higher disability risk due to more frequent optic nerve and spinal cord involvement compared to Caucasians. Today, anti-AQP4 antibody testing facilitates the differentiation of the Asian form of MS from NMOSD (Wingerchuk et al. 2015).
Pediatric NMOSD
Approximately 4% of NMOSD cases are reported to have a pediatric onset. Early differentiation of NMOSD from other childhood demyelinating disorders including MS and acute disseminated encephalomyelitis (ADEM) is critical for instituting the appropriate therapy. However, presence of anti-AQP4 antibodies is less frequent in pediatric NMOSD compared to adult onset NMOSD, hampering the diagnosis. A diagnostic algorithm is presented in Fig. 2 (Hacohen et al. 2017). All characteristic MRI features of NMOSD including LETM, area postrema and medullary lesions, hypothalamic, callosal, and periaqueductal lesions have been described in pediatric onset NMOSD. Lesions tend to be more destructive in comparison to pediatric MS or MOG-related disease. Involvement of the conus of the spinal cord is uncommon. In general, children across all antibody-mediated neuroinflammatory conditions show more frequent bilateral, large, brainstem, and deep gray matter lesions compared to adults (Jurynczyk et al. 2017).
Diagnostic algorithm for CNS demyelination in children. (Modified from Hacohen et al. 2017)
Anti-MOG-Ab-Related Disease
The presence of antibodies directed against myelin oligodendrocyte glycoprotein (MOG) has been reported in a subgroup of patients with NMOSD (with negative anti-AQP4 antibodies) and ON and a limited number of MS patients. These anti-MOG antibodies – predominantly the IgG1 isoform – are directed against extracellular epitopes of MOG on the surface of the myelin sheath and associated with CNS demyelinating syndromes, particularly in pediatric acute disseminated encephalomyelitis (ADEM)-like disease onset. Adults and children with MOG-abs often present with few (<3) fluffy brainstem lesions, often located in the pons, middle cerebellar peduncle, or close to the fourth ventricle. Blood-brain barrier breakdown visualized by contrast enhancement on MRI is frequent.
Although initially reported as a monophasic condition (often associated with ADEM), MOG-abs-related disease appears to be relapsing in 50% of all cases. The majority of patients with MOG-abs, lesions showed partial or complete resolution on follow-up MRI (Hacohen). Similar to NMOSD, ON and LETM are frequent findings in MOG-related disease; a typical feature is involvement of the conus. However, in contrast to AQP4-IgG-positive NMOSD, there is frequent occurrence of seizures with corresponding cortical and subcortical MRI findings in MOG patients.
Diagnostic Criteria
The 2015 criteria devise a distinction between NMOSD with AQP4-IgG and NMOSD without AQP4 abs or with unknown antibody status (Wingerchuk et al. 2015). In case of a positive AQP4-IgG serostatus (using best available detection method, cell-based assay strongly recommended and exclusion of alternative diagnoses (Geraldes et al. 2018)), an NMOSD diagnosis can be established if one of the core clinical core symptoms is present (Table 2).
By contrast, the diagnosis of NMOSD without AQP4-IgG or with unknown antibody status requires fulfillment of additional criteria which often involve typical MRI abnormalities (Table 3).
Imaging Strategy
The most common setting for imaging is a female patient with transverse myelitis or bilateral optic neuritis but sometimes specific brainstem syndromes such as intractable hiccup. The purpose of the MRI examination is to confirm the site of involvement and search for findings supporting a diagnosis of NMOSD or an alternative cause (e.g., MS, sarcoid). The MRI protocol is therefore similar to demyelination, include contrast administration, and cover the brain, optic nerve, and spinal cord (Table 4).
MRI Features in NMOSD by Region of Involvement
Spinal Cord
Longitudinally extensive transverse myelitis (LETM) is defined as a lesion extending over three or more vertebrae in cranio-caudal direction. These lesions show a continuous signal increase in T2-weighted (T2w), proton density (PD), or short tau inversion recovery (STIR) sequences often with patchy or ring-like enhancement (Fig. 3). In case of severe disease, LETM can be associated with T1-weighted (T1w) signal decrease of the gray matter (“pseudo-syringomyelia”) (Fig. 4).
NMO lesions have a central cord predominance with more than 70% of the lesion residing within the central gray matter (Wingerchuk et al. 2015) and local cord swelling/expansion. This is in contrast to MS lesions, which usually span less than one vertebral segment, are often multiple, and are commonly peripherally located within the white matter, although gray mater involvement can also be seen. Short segment cord lesions may also be seen in early (seropositive NMOSD) but may be more ill-defined. Furthermore, LETM lesions may evolve into multiple shorter lesions during remission or after treatment with steroids (Kim et al. 2015). Hence, the timing of spinal cord MRI in relation to the onset of symptoms is critical for the identification of a characteristic LETM presentation (Geraldes et al. 2018).
Although the LETM pattern is characteristic of NMOSD, 7–14% of initial and 8% of subsequent myelitis attacks in AQP4 seropositive patients do not meet the LETM definition (Wingerchuk et al. 2015; Flanagan et al. 2015). On the other hand, some progressive MS patients may have coalescent cord lesions that can superficially suggest a LETM pattern. Therefore, NMOSD must be considered in the differential diagnosis in patients presenting with short myelitis lesions, and appearance on both the sagittal and axial plane should be considered to judge the lesion extent and architecture. On axial T2w, “bright spotty lesions” can be seen in NMOSD, reflecting microcystic changes of the spinal cord (Yonezu et al. 2014) (Figs. 5 and 6).
Contrast enhancement on T1w images is present in the majority of acute LETM in NMOSD, detectable for days to months following relapse and can be ring-like or patchy (Fig. 7). Lesions may disappear almost entirely during remission. However, pronounced and extensive spinal cord atrophy with or without T2w hyperintensity can occur (Geraldes et al. 2018).
The differential diagnosis of LETM (Table 5) include CNS sarcoidosis, acute disseminated encephalomyelitis (ADEM), spinal cord infarction, dural arteriovenous fistula, systemic vasculitis, vitamin B12 deficiency, spondylotic myelopathy, or rarely MS. LETM seems to be less specific for pediatric NMOSD, as in children it is frequently observed also in ADEM, in pediatric MS and in children with monophasic transverse myelitis (Kim et al. 2015).
The most important differential diagnosis of NMOSD remains MS, and several imaging features can help in the distinction (Table 6), mostly based on the appearances on T2w images and contrast enhancement pattern.
Optic Nerve
In more than 50% of NMOSD patients, optic neuritis (ON) is the presenting symptom, often bilateral. Orbital MRI performed early and prior to treatment initiation can facilitate the diagnosis, in absence of dissemination in space and if spinal and brain MRI are normal. For optic nerve imaging, fat-suppressed sequences should be applied in a coronal image plane. During the acute phase of ON, optic nerve sheath thickening, unilateral or bilateral T2w hyperintensity, and T1w contrast enhancement within the affected optic nerve and/or optic chiasm can be seen (Fig. 8).
In NMOSD, optic nerve lesions are often longer than in MS with posterior extension into the optic chiasm (Figs. 9 and 10) suggesting AQP4 and anterior extension or optic nerve head swelling favoring MOG antibodies tends to occur in MOG-ON, but not in MS-ON.
A 35-year-old woman with NMO presenting with acute bilateral visual loss. Coronal fat-suppressed T2w images demonstrate thickening and hyperintensity of both optic nerves, with involvement of optic chiasm (yellow arrows). Note marked bilateral enhancement, more marked on the left side on the coronal fat-suppressed contrast-enhanced T1w image (red arrow)
Brain
Though classically considered a disease NOT to involve the brain by definition, it has become evident that cerebral involvement in NMO is certainly not an exception (Kim et al. 2011), though may be absent at disease onset. In patients with positive AQP4-antibody testing, development of cerebral lesions is quite common during the clinical course of NMOSD (Kim et al. 2015). The 2015 NMOSD diagnostic criteria describe the characteristic brain parenchymal lesion locations in areas of high AQP4 expression frequency (Table 7).
In contrast to, e.g., MS, cortical lesions seem to be rare or absent in NMOSD (Kim et al. 2015), as are lesions in Dawson fingers configuration and white matter lesions with a central vein, both of which are characteristic for MS.
Tumefactive lesions are quite typical of NMOSD (Fig. 11) (Zabad et al. 2017), and in fact when large hemispheric lesions are seen in a patient suspected of MS, antibody testing should be performed. As already discussed in the MS chapter, its rare variant Baló’s concentric sclerosis is characterized by alternating rings of demyelinated and preserved myelin layers. The occurrence of tumefactive lesions with concentric rings of Baló has also been described in NMOSD patients. Although selective loss of AQP4 and vasculocentric deposition of complement and immunoglobulins are characteristic features of NMOSD, it has been shown that extensive AQP4 loss and perivascular lymphocytic cuffing of T cells is present in alternating layers of myelinated and demyelinated Baló-like NMOSD lesions; however, no deposition of immunoglobulins or complement around vessels was detectable. This finding suggests that AQP4 loss may occur in various demyelinating conditions, namely, Baló and NMOSD, but does not seem to be a specific feature of NMOSD.
Large hemispheric lesions in NMO affecting subcortical white matter and centrum-semiovale, the cortical spinal tract including posterior limb internal capsule , and corpus callosum involvement. (Reproduced with permission from Kim et al. 2012)
Treatment
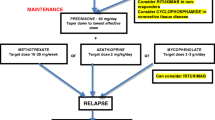
Acute relapses are usually treated with high-dose intravenous steroids (IVMP) as in MS. Patients who do not adequately respond to a first treatment course with IVMP can receive further early treatment with plasma exchange or immune absorption. Especially myelitis attacks may benefit from immediate plasma exchange, and a timely initiation of this therapeutic intervention is crucial for its success.
As long-term disability results from incomplete remission of attacks, prevention of further relapses is the goal of preventative immunotherapy in NMOSD. Most MS immunomodulatory drugs such as beta interferon, glatiramer acetate, natalizumab, fingolimod, and alemtuzumab (and presumably dimethyl fumarate) are ineffective or even deleterious in NMOSD and therefore contraindicated. By contrast, immunosuppressive B-cell depleting drugs such as azathioprine, mycophenolate mofetil (MMF), oral prednisolone, and rituximab reduce relapse rates by 50–80%. Rituximab is considered the most efficacious attack-preventing drug in AQP4-ab positive NMOSD. In recent years, several randomized controlled clinical trials with compounds aimed at targeting immunopathogenesis of the disease have been launched. In anti-MOG-Ab-related disease, preventative treatment data are even more scarce. Case series suggest a favorable response to oral steroids with a high risk of relapse when tapered off (at least in seropositive patients) and a similar lack of efficacy of interferon-beta. Many MOG-ab positive patients respond well to rituximab, albeit possibly at a lower success rates than AQP4-IgG positive NMOSD.
Structured Reporting
The report at onset should state anatomical and quantitative details (location, number, and size of lesions) and qualitative information (extent and configuration of T2w/FLAIR hyperintensity, contrast enhancement) on the presence of pathology in the three main CNS regions: the spinal cord, brain, and optic nerves. For follow-up examinations, the evolution of T2w/FLAIR hyperintensity, continuation or absence of contrast enhancement, as well as development of (cord/optic) atrophy should be reported.
Checklist for Reporting: Features that Should Raise the Possibility of NMO
Spine
-
LETM (extending more than three vertebral segments)
-
Ring-enhancing or patchy contrast enhancement
-
Microcystic or heterogeneous appearance on T2w MRI
-
Focal cord atrophy
-
Conus involvement (MOG)
Brain
-
Atypical, large hemispheric lesions
-
Corpus callosum involvement
-
Peri-ependymal brainstem lesions
-
Dorsal medulla/area postrema lesions
Optic Nerve
-
Bilateral involvement
-
Extensive contrast enhancement
-
Chiasm involvement (AQP4)
-
Optic nerve atrophy (follow-up)
Sample Report
History
A 41-year-old woman with a history of optic neuropathy and a mixed connective tissue disorder with seropositive antinuclear antibodies. Recent development of symptoms of transverse myelitis
MRI protocol
Sagittal and axial T2w and contrast enhanced T1w MRI, no prior examination for comparison.
Findings
Normal craniocervical junction and area postrema. There is a large area of T2w-hyperintensity spanning form C2 to C6 with ill-defined borders with preferential involvement of the central part of the cord. The cord appears swollen in the affected segment. After contrast agent administration, there is patchy, partly rim-lime, enhancement (Fig. 12).
Interpretation
Longitudinally extensive transverse myelitis (LETM), given previous history consistent with NMO
Abbreviations
- ADEM:
-
Acute disseminated encephalomyelitis
- AQP4:
-
Aquaporin-4 channel
- CNS:
-
Central nervous system
- Fat-sat:
-
Fat saturation
- Gad:
-
Gadolinium
- GFAP:
-
Glial fibrillary acidic protein
- IgG:
-
Immunoglobulin-G
- IVMP:
-
High-dose intravenous steroids
- LEON:
-
Longitudinally extensive optic neuritis
- LETM:
-
Longitudinally extensive transverse myelitis
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- NMOSD:
-
Neuromyelitis spectrum disorder
- ON:
-
Optic neuritis
- STIR:
-
Short tau inversion recovery
- T2w:
-
T2-weighted
References
Ciccarelli O, Cohen JA, Reingold SC, Weinshenker BG. Spinal cord involvement in multiple sclerosis and neuromyelitis optica spectrum disorders. International Conference on Spinal Cord Involvement and Imaging in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders. Lancet Neurol. 2019;18(2):185–197.
Flanagan EP, Weinshenker BG, Krecke KN, Lennon VA, Lucchinetti CF, McKeon A, Wingerchuk DM, Shuster EA, Jiao Y, Horta ES, Pittock SJ. Short myelitis lesions in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. JAMA Neurol. 2015;72(1):81–7.
Geraldes R, Ciccarelli O, Barkhof F, De Stefano N, Enzinger C, Filippi M, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol. 2018;14:199.
Hacohen Y, Mankad K, Chong W, Barkhof F, Vincent A, Lim M, et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology. 2017;89:269–78.
Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140:617–27.
Kim W, Kim S-H, Hyun Lee S, Feng Li X, Jin Kim H. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler J. 2011;17(9):1107–12.
Kim W, Kim SH, Huh SY, Kim HJ. Brain abnormalities in neuromyelitis optica spectrum disorder. Mult Scler Int. 2012;2012:735486.
Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84:1165–73.
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–9.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89.
Yonezu T, Ito S, Mori M, Ogawa Y, Makino T, Uzawa A, et al. “Bright spotty lesions” on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult Scler. 2014;20:331–7.
Zabad R, Stewart R, Healey KM. Pattern recognition of the multiple sclerosis syndrome. Brain Sci. 2017;7(10):138.
Suggested Reading
Dubey D, Pittock SJ, Krecke KN, Morris PP, Sechi E, Zalewski NL, et al. Clinical, radiologic, and prognostic features of myelitis associated with myelin oligodendrocyte glycoprotein autoantibody. JAMA Neurol 2018. [Epub ahead of print].
Dutra BG, da Rocha AJ, Nunes RH, Júnior MACM. Neuromyelitis optica spectrum disorders: spectrum of MR imaging findings and their differential diagnosis. Radiographics. 2018;38:169–19.
Huh S-Y, Min J-H, Kim W, Kim S-H, Kim HJ, Kim B-J, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Scler J. 2014;20:695–704.
Matthews L, Marasco R, Jenkinson M, Küker W, Luppe S, Leite MI, et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology. 2013;80:1330–7.
Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite M, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler J. 2015;21:845–53.
Weinshenker BG, Wingerchuk DM, editors. Neuromyelitis spectrum disorders. Mayo Clin Proc. 2017;92:663–679.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Wuerfel, J., Rovira, À., Paul, F., Barkhof, F. (2019). Neuromyelitis Optica Spectrum Disorders (NMOSD). In: Barkhof, F., Jäger, H., Thurnher, M., Rovira, À. (eds) Clinical Neuroradiology. Springer, Cham. https://doi.org/10.1007/978-3-319-68536-6_71
Download citation
DOI: https://doi.org/10.1007/978-3-319-68536-6_71
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68535-9
Online ISBN: 978-3-319-68536-6
eBook Packages: MedicineReference Module Medicine