Abstract
There are several consensus criteria for both the clinical and neuropathological diagnosis of different types of dementias. The clinical diagnostic accuracy using revised research criteria and newly developed biomarkers (MRI, PET, CSF analysis, genetic markers) ranges from 65 to 96% (for Alzheimer disease) with a specificity of diagnostic criteria versus other dementias of 23–88%. Neuropathological assessment of dementing disorders using immunohistochemistry, molecular biologic and genetic methods can achieve a diagnosis/classification, based on the homogeneous definitions, harmonized inter-laboratory methods and standards for the assessment of nervous system lesions, in about 99%, without, however, being able to clarify the causes/etiology of most of these disorders. Further prospective and concerted clinicopathological studies using revised methodological and validated protocols and uniform techniques are required to establish the nature, distribution pattern and grades of lesions and; thus, to overcome the limitations of the current diagnostic framework. By data fusion this my allow their more uniform application and correlation with the clinical data in order to approach a diagnostic “gold standard”, and to create generally accepted criteria for differentiating cognitive disorders from healthy brain aging. The detection of disease-specific pathologies will be indispensable to determinate the efficacy of new therapy options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia encompasses deteriorations in several cognitive domains [8, 36, 103], see [40]; it has been re-defined as the differential manifestation of deteriorating brain functions over time as a part of aging due to cell deaths in the brain caused by neurodegeneration or any other disease [117]. However, because of recent research data, dementia is not only caused by ‘neuronal cell death’/cell loss [91, 104, 151], but predominantly by dysfunction and loss of synapses [43, 60, 128, 129, 140, 141] that have been demonstrated in early Alzheimer disease (AD) [123] and in dementia with Lewy bodies (DLB) [83], and by cholinergic neuronal and axonal abnormalities that also are present in aging and AD [48]. These changes cause disconnections of important nervous circuitries [14, 35, 68, 114] that have been demonstrated in vivo in early AD [152].
The aim of this review is to discuss the diagnostic validity of currently used neuropathological criteria particularly of neurodegenerative dementias and their limitations as well as to give recommendations for future clinicopathological research.
Research and consensus criteria for the clinical diagnosis of the major dementing disorders exist and have recently been revised, e.g., the NINCDS-ADRDA and DSM-IV-TR criteria for AD [36, 37], Parkinson disease dementia (PDD) [42], DLB [46, 97], frontotemporal lobe degeneration (FTLD) [77, 90, 109], vascular dementia or vascular cognitive disorder (SCADDTD [26]; NINDS-AIREN [106, 125, 126, 155]), mixed dementias [71], and others. Although the NINCDS-ADRDA criteria [98] combined with neuropsychologic assessment are still valid for 88–90% of AD [80], they do not exclude additional Lewy body and other pathologies [122]. Combination of clinical data with the fusion of different biomarkers has already improved the clinical diagnostic accuracy of AD up to 96%, while their sensitivity and specificity versus other dementias is still lower. A combination of the best CSF and MRI data will lead to a more precise diagnostic prediction [30].
Advantages and pitfalls of neuropathological diagnostic criteria
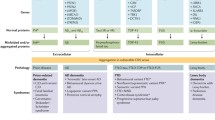
At present, several diagnostic guidelines for the neuropathological diagnosis of most dementing disorders are used, relying on the qualitative, semiquantitative, and topographic assessment of morphological and bio/histochemical sign posts, in particular specific protein inclusions in neurons, glia and other cells. The neuropathology of dementia has been suggested to appear as a “science of inclusion bodies” [39], see also [40]. A classification of degenerative dementias based on currently known biochemical markers is given in Table 1.
For α-synucleinopathies, in particular Lewy body disorders, in addition to assessment criteria [5], staging/classification systems based on the semiquantitative assessment of the distribution and progression pattern of α-synuclein (αSyn) pathology are used that are suggested to indicate a predictable sequence of lesions [22, 24]. They allow a distinction of three major phenotypes of LB disease—brainstem predominant, transitional/limbic and diffuse cortical [89, 97], while AD with amygdala Lewy bodies is considered a distinct form of α-synucleinopathy [147]. Recent clinicopathologic studies, although partly confirming these systems, have shown that between 6.3 and 47% of cases did not follow the proposed staging pattern of αSyn pathology [75, 76, 116]. On the other hand, 30–55% of elderly subjects with widespread/cortical αSyn pathology revealed no definite neuropsychiatric symptoms or were not classifiable [1, 17, 71, 72, 86, 159], and the criteria for categorization of Lewy-related pathology in patients with dementia had to be modified [1, 46, 86]. PDD and DLB, sharing many clinical and morphological features and believed to form a continuum within the spectrum of LB diseases [11, 55, 97], have been shown to differ by more severe diffuse amyloid load in the striatum of DLB [69, 87], recently confirmed by in vivo studies using 11CPIB-PET [41, 54], as well as by more frequent LB affection of the hippocampal CA 2–3 subareas [76]. The predictive value of striatal Aβ pathology, with regard to cognitive impairment is still controversial [1].
For FTLD, nowadays suggested to be the third or forth most frequent cause of dementias, criteria for neuropathological diagnosis are based on the basic biochemical markers [25, 77, 90, 105] including the novel TDP-43 neurodegenerative proteinopathies [45]. Recently, a small novel group of FTLD patients with clinical features that overlap with DLB has been identified, which morphologically were consistent with TDP-43 proteinopathy (FTLD with ubiquitin-only lesions, type I) [29, 149].
Despite various proposals for a categorization of major cerebrovascular lesions [78], a harmonization of the criteria and techniques for the assessment of cerebral lesions of presumable/possible vascular origin in cognitively impaired is necessary [115]. Because of its high variability of morphological findings and multifactorial pathogenesis of vascular cognitive impairment, no generally accepted morphologic scheme for quantitating cerebrovascular lesions and no validated neuropathological criteria for vascular dementia have been established to date (see [70]). The same holds for so-called “mixed dementia” [71, 73]. Recently, BrainNet Europe II (BNE) has constituted a “vasculopathy” reference group to harmonize the assessment of vascular pathology similar to previous interlaboratory studies for the morphological assessment of AD-related and αSyn pathologies [5, 6, 23].
In contrast to most dementing conditions that typically develop over years, rapidly progressive dementia being quickly fatal, is one of the most challenging neuropathological problems. The differential diagnosis is often widely ranging, and in addition to frequent sporadic or genetic prion diseases includes rapidly progressing neurodegenerative tauopathies and synucleinopathies, autoimmune conditions infectious, toxic-metabolic and neoplastic diseases [47]. In these and other unclear conditions, brain biopsy may play a role, although frequent biopsy findings in dementia are non specific [153].
Specific problems in the diagnosis of AD
Histopathological examination of the brain establishes that AD-related lesions are present in sufficient densities to distinguish AD from age-related lesions and allows detection of other dementing disorders [39]. Although the interlaboratory comparison of neuropathological assessment of AD when using standardized criteria showed reasonable interrater agreement [3, 4, 6, 13, 38, 56, 95, 102, 108, 109], no one set of histopathological criteria for AD has been uniformly accepted by neuropathologists [156]. These algorithms that only consider the classical “plaque and tangle” phenotype of AD do not recognize other dementias and AD subtypes, e.g., the “plaque predominant” type with abundant amyloid plaques, no or very little neuritic pathology restricted to the hippocampus and abnormal phosphorylated tau in neocortical pyramidal cells but lacking overt tangle formation, accounting for 3.5–8% of demented subjects over age 85 years [66, 139, 144, 145], the “tangle dominant type” with 3+4R tau pathology often restricted to the limbic system, absence of neuritic plaques and no or very little amyloidosis, accounting for 5–7% of oldest-olds [73], and the Lewy body variant of AD, with cortical and subcortical Lewy bodies, often associated with severe AD pathology [57]. Recent studies showed different cytoskeletal alterations in familial AD (FAD) due to PSEN1 mutations and sporadic AD [157]. Furthermore, since standard neuropathological metrics for tangles and neuritic plaques [21, 62, 102] are usually semiquantitative and, according to the BNE consortium, good agreement can be reached in the neuropathological diagnosis only when the lesions are substantial, e.g., when they have reached isocortical structures (Braak stage V–VI with absolute agreement 91%). In contrast, for mild lesions the agreement was poorer (Braak stage I–II, agreement 50%) [6], thereby limiting the ability to make accurate correlation of antemortem cognitive status and the severity of morphological findings. Although the sensitivity and specificity of the NIA-RI criteria is suggested to be 90%, only 40–57% of the brains of patients with the clinical diagnosis of probable AD show “pure” AD pathology (Table 4). Thus, their predictive value may be reduced to 38–44% [20].
Diffuse and neuritic plaques and some amounts of tau-positive neuritic pathology in the limbic system relatively frequently occur in cognitively normal elderly [10, 15, 18, 32, 34, 61, 68, 81, 120, 130]. Although cognitively unimpaired subjects may show some or even considerable AD-related pathology [94, 137, 138], in general, the number of isocortical tangles correlates best with clinical dementia severity [12, 15, 19, 92, 110, 131, 144, 145], and patterns of gray matter loss associated with tangle pathology is an appropriate in vivo surrogate indicator of AD pathology [154]. The predictive value of widespread tau pathology (Braak stages V–VI) for dementia is high [1], while others found that both diffuse and neuritic plaques, rather than tangles in neocortical regions distinguish non-demented and AD subjects with high sensitivity and specificity [96].
Neuropathology of AD in dementia in the oldest-old differs considerably in both intensity and distribution from that in younger age groups [49, 50, 150]. Significantly increased densities of neuritic plaques and NFTs are absent in demented patients over age 85–90 years [52, 59, 64, 110, 119, 121, 136], and there is a considerable overlap in the pathologies found in the demented and non-demented [158]. A high percentage of demented aged persons aged 80+ do not meet the pathological criteria of AD or were classified as “dementia of unknown etiology” [33, 65].
Another important point is the frequent presence of confounding processes in the aged brain that coexist with AD, e.g., cerebrovascular disease, Lewy body pathology, argyrophilic grain disease, hippocampal sclerosis etc, about two-thirds of aged human brains containing non-AD-type neuropathology [107, 110]. The frequency of mixed pathologies in demented elderly is shown in Table 4, and was recently confirmed in over 3,300 autopsy cases of aged demented individuals [82]. Since 50–85% of the brains of persons who die aged 80–90+ old show appreciable cerebrovascular lesions [118], a specific problem is the impact of cerebrovascular disease in relation to AD pathology [16, 27, 28, 51, 110, 134, 146]. The burden of vascular and AD type lesions are considered to be independent of each other, and are consistent with an additive or synergistic effect of both types of lesions on cognitive impairment [67, 71, 72, 84, 132]. The thresholds for vascular and degenerative lesions in distinguishing “pure” VaD or AD from mixed cases have been critically discussed recently [53, 160]. AD pathology alone more frequently accounts for dementia than both microscopic and macroscopic infarcts [146], and in advanced or full-blown stages of AD concomitant small vascular lesions do not significantly influence the overall state and progression of cognitive decline, the severity and extent of AD pathology overwhelming the effects of cerebrovascular disease [27, 71–73, 85]. Nevertheless, it should be borne in mind that all additional pathologies may interact. Therefore, the reliability and clinical relevance of the current diagnostic criteria need better qualification and validation.
Conclusions
Adequate use of morphological markers (tau, amyloidosis, synuclein, synaptic and other proteins) as well as biochemical markers usually identify the vast majority of cases with dementia. However, due to variable overlap, these changes may fail to distinguish between cognitively intact aged subjects from those with mild cognitive impairment or preclinical or mild AD. In particular, these latter groups show a wide variety in the intensity and pattern of AD-related lesions. Although they often differ from “normal” aging, only a small proportion of cognitively intact aged are free of AD pathology, while up to 50% may show AD-related changes or even definite AD pathology [10, 15, 18, 32, 34, 61, 68, 81, 120, 130]. Additional difficulties arise from frequent coexistence with other pathologies that may have an additive or synergistic effect. Similar difficulties arise in other neurodegenerative dementias, in particular those with genetic background. The question, whether the neuropathological “gold standard” is dead has been discussed in respect to genetical neurodegenerative disorders that offer a rare opportunity to test the validity of neuropathological diagnostic criteria. Implications regarding an autosomal dominant disorder (PARK 8) in which four different morphological diagnoses were found at autopsy were discussed. It was suggested that just as there is currently no clinical “gold standard” for Parkinson disease, in certain circumstances there is also no pathological “gold standard”, and only the combination with genetic studies may provide definitive arbitration of validity of clinical and pathologic diagnostic criteria [148, 149]. In view of these difficulties, the present author feels not competent enough to present or propose “new” criteria for the neuropathological diagnosis of neurodegenerative and dementing disorders and, thus, to show a “simple” way out of the current “chaos” regarding histological diagnosis of dementia and their clinical implications. Further studies using methodological and validated protocols and harmonized techniques are required to increase the accuracy and reproducibility of neuropathological diagnosis. Keeping all the pros and cons in mind, one has to conclude that, if robust correlations between clinical course and morphological changes will be confirmed by future correlative studies, the neuropathological classification and staging systems will need to be revised accordingly. There will be a long and difficult way out of the swamp.
References
Aho L, Parkkinen L, Pirttila T, Alafuzoff I (2008) Systematic appraisal using immunohistochemistry of brain pathology in aged and demented subjects. Dement Geriatr Cogn Disord 25:423–432
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65:1509–1517
Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopoulou P, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2006) Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe consortium. J Neuropathol Exp Neurol 65:740–757
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18:484–496
Alafuzoff I, Parkkinen L, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Morris J, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2008) Assessment of alpha-synuclein pathology: a study of the BrainNet Europe consortium. J Neuropathol Exp Neurol 67:125–143
Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H (2008) Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol 115:533–546
Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H, BrainNet E (2009) Inter-laboratory comparison of neuropathological assessments of beta amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol (in press)
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. Text Revision, 4th edn. American Psychiatric Association, Washington DC
Andin U, Gustafson L, Passant U, Brun A (2005) A clinico-pathological study of heart and brain lesions in vascular dementia. Dement Geriatr Cogn Disord 19:222–228
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Ballard C, Ziabreva I, Perry R, Larsen JP, O’Brien J, McKeith I, Perry E, Aarsland D (2006) Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 67:1931–1934
Bancher C, Jellinger K, Lassmann H, Fischer P, Leblhuber F (1996) Correlations between mental state and quantitative neuropathology in the Vienna longitudinal study on dementia. Eur Arch Psychiatry Clin Neurosci 246:137–146
Bancher C, Paulus W, Paukner K, Jellinger K (1997) Neuropathologic diagnosis of Alzheimer disease: consensus between practicing neuropathologists? Alzheimer Dis Assoc Disord 11:207–219
Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL (2004) Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging 25:843–851
Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE (2004) Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 61:378–384
Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS (2005) Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 64:834–841
Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66:1837–1844
Berg L, McKeel DW Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM (1998) Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 55:326–335
Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP (1995) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 52:81–88
Bowler JV, Munoz DG, Merskey H, Hachinski V (1998) Fallacies in the pathological confirmation of the diagnosis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 64:18–24
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol (Berl) 112:389–404
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CLIII, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol (Berl) 114:5–22
Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R (1992) Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s disease diagnostic and treatment centers. Neurology 42:473–480
Chui HC (2006) Vascular cognitive impairment: today and tomorrow. Alzheimers Demen 2:185–194
Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, Mungas D, Reed BR, Kramer JH, Decarli CC, Weiner MW, Vinters HV (2006) Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol 60:677–687
Claassen DO, Parisi JE, Giannini C, Boeve BF, Dickson DW, Josephs KA (2008) Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol 21:157–163
Clark CM, Davatzikos C, Borthakur A, Newberg A, Leight S, Lee VM, Trojanowski JQ (2008) Biomarkers for early detection of Alzheimer pathology. Neurosignals 16:11–18
Cochran EJ, Schneider JA, Bennett DA et al (1998) Application of NIA/Reagan Institute Working Group Criteria for diagnosis of Alzheimer’s disease to members of the religious orders study (abstr.). J Neuropathol Exp Neurol 57:508
Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L (1988) Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38:1682–1687
Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB (2000) The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol 57:713–719
Davis DG, Schmitt FA, Wekstein DR, Markesbery WR (1999) Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58:376–388
Delatour B, Blanchard V, Pradier L, Duyckaerts C (2004) Alzheimer pathology disorganizes cortico-cortical circuitry: direct evidence from a transgenic animal model. Neurobiol Dis 16:41–47
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Duyckaerts C, Delaère P, Hauw JJ, Abbamondi-Pinto AL, Sorbi S, Allen I, Brion JP, Flament-Durand J, Duchen L, Kauss J, Schlote W, Lowe J, Probst A, Ravid R, Swaab DF, Renkawek K, Tomlinson B (1990) Rating of the lesions in senile dementia of the Alzheimer type: concordance between laboratories. A European multicenter study under the auspices of EURAGE. J Neurol Sci 97:295–323
Duyckaerts C (2008) Neuropathologic classification of dementias: introduction. In: Duyckaerts C, Litvan I (eds) Handbook of clinical neurology, vol 89 (3rd series). Elsevier, Edinburgh, pp 147–159
Duyckaerts C, Litvan I (2008) Handbook of clinical neurology, vol 89 (3rd series). Edinburgh, Elsevier
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Nagren K, Chaudhuri R, Masters CL, Brooks DJ (2008) Amyloid load in Parkinson’s disease dementia and Lewy Body dementia measured with [11C]PIB-PET. J Neurol Neurosurg Psychiatry (in press). doi:10.1136/jnnp.2007.127878
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Fein JA, Sokolow S, Miller CA, Vinters HV, Yang F, Cole GM, Gylys KH (2008) Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol 172:1683–1692
Fernando MS, Ince PG (2004) Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci 226:13–17
Forman MS, Trojanowski JQ, Lee VM (2007) TDP-43: a novel neurodegenerative proteinopathy. Curr Opin Neurobiol 17:548–555
Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW (2008) Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol 67:649–656
Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL (2008) Rapidly progressive dementia. Ann Neurol 64:97–108
Geula C, Nagykery N, Nicholas A, Wu CK (2008) Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol 67:309–318
Giannakopoulos P, Hof PR, Giannakopoulos AS, Herrmann FR, Michel JP, Bouras C (1995) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol 52:1150–1159
Giannakopoulos P, Hof PR, Kovari E, Vallet PG, Herrmann FR, Bouras C (1996) Distinct patterns of neuronal loss and Alzheimer’s disease lesion distribution in elderly individuals older than 90 years. J Neuropathol Exp Neurol 55:1210–1220
Giannakopoulos P, Gold G, Kovari E, von Gunten A, Imhof A, Bouras C, Hof PR (2007) Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol 113:1–12
Gold G, Bouras C, Kovari E, Canuto A, Glaria BG, Malky A, Hof PR, Michel JP, Giannakopoulos P (2000) Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol (Berl) 99:579–582
Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E (2007) Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 130:2830–2836
Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, Hyman BT, Growdon JH, Johnson KA (2008) Imaging amyloid deposition in Lewy body diseases. Neurology 71:903–910
Gross RG, Siderowf A, Hurtig HI (2008) Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies: a spectrum of disease. Neurosignals 16:24–34
Halliday G, Ng T, Rodriguez M, Harding A, Blumbergs P, Evans W, Fabian V, Fryer J, Gonzales M, Harper C, Kalnins R, Masters CL, McLean C, Milder DG, Pamphlett R, Scott G, Tannenberg A, Kril J (2002) Consensus neuropathological diagnosis of common dementia syndromes: testing and standardising the use of multiple diagnostic criteria. Acta Neuropathol (Berl) 104:72–78
Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M et al (1990) The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 40:1–8
Harding AJ, Kril JJ, Halliday GM (2000) Practical measures to simplify the Braak tangle staging method for routine pathological screening. Acta Neuropathol 99:199–208
Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, Libow LS, Lesser GT, Maroukian M, Grossman HT (2008) Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol 65:1211–1217
Hu NW, Smith IM, Walsh DM, Rowan MJ (2008) Soluble amyloid-beta peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain 131:2414–2424
Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM (1998) Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol 57:1168–1174
Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097
ICDNS, Duyckaerts C (2003) Alzheimer disease diagnosis. http://www.icdns.org/forums/index.php?showtopic=27
Imhof A, Kovari E, von Gunten A, Gold G, Rivara CB, Herrmann FR, Hof PR, Bouras C, Giannakopoulos P (2007) Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci 257:72–79
Jellinger KA (2001) Frequency of “dementia of unknown origin” increases with age (Letter). Arch Neurol 58:1498–1499
Jellinger KA (2003) Plaque-predominant and tangle-predominant variants of Alzheimer’s disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press, Basel, pp 66–68
Jellinger KA, Attems J (2005) Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer’s disease. J Neurol Sci 229–230:37–41
Jellinger KA (2006) A view on early diagnosis of dementias from neuropathology. In: Herholz K, Morris C, Perani D (eds) The dementias: early diagnosis and evaluation. Taylor & Francis, New York, pp 311–428
Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112:253–260
Jellinger KA (2007) Lewy body disorders. In: Youdim MBH, Riederer P, Mandel SA, Battistin L, Lajtha A (eds) Degenerative diseases of the nervous system. Springer, New York, pp 267–343
Jellinger KA (2007) The enigma of mixed dementia. Alzheimer Demen 3:40–53
Jellinger KA (2007) The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol (Berl) 113:349–388
Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257:80–87
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117
Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116:1–16
Jellinger KA (2009) Significance of brain lesions in Parkinson’s disease dementia and Lewy body dementia. In: Giannakopoulos P, Hof P (eds) Front Neurol Neurosci: Dementia in Clinical Practice. Karger, Basel, pp 1–12 (in press)
Josephs KA (2008) Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol 64:4–14
Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226:75–80
Khachaturian ZS (1985) Diagnosis of Alzheimer’s disease. Arch Neurol 42:1097–1105
Klatka LA, Schiffer RB, Powers JM, Kazee AM (1996) Incorrect diagnosis of Alzheimer’s disease. A clinicopathologic study. Arch Neurol 53:35–42
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Kovacs GG, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Capellari S, Ferrer I, Gelpi E, Kovari V, Kretzschmar H, Nagy Z, Parchi P, Seilhean D, Soininen H, Troakes C, Budka H (2008) Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord 26:343–350
Kramer ML, Schultz-Schaeffer WJ (2007) Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27:1405–1410
Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR (2008) AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging 29:1587–1590
Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ (2000) Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch Neurol 57:1474–1479
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ (2008) Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 18:220–224
Liang T, Noorigian J, Duda JE (2006) Does striatal pathology distinguish DLB from PDD? (abstr). Mov Disord 21(suppl 13):S69–S70
Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, Bowen J, Teri L, Thompson J, Peskind ER, Raskind M, Larson EB (1999) Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc 47:564–569
Lowe J (2008) Neuropathology of dementia with Lewy bodies. In: Duyckaerts C, Litvan I (eds) Handbook of clinical neurology, vol 89 (3rd series). Elsevier, Edinburgh, pp 321–330
Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Mann DM (1996) Pyramidal nerve cell loss in Alzheimer’s disease. Neurodegeneration 5:423–427
Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR (2006) Neuropathologic substrate of mild cognitive impairment. Arch Neurol 63:38–46
McKee AC, Kowall NW, Au R (2002) Topography of neurofibrillary tangles distinguishes aging from Alzheimer disease (abstr.). J Neuropathol Exp Neurol 61:488
McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, Drake J, Wolf PA (2006) Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol 65:621–630
McKeel DW Jr, Ball MJ, Price JL, Smith DS, Miller JP, Berg L, Morris JC (1993) Interlaboratory histopathologic assessment of Alzheimer neuropathology: different methodologies yield comparable diagnostic results. Alzheimer Dis Assoc Disord 7:136–151
McKeel DW Jr, Price JL, Miller JP, Grant EA, Xiong C, Berg L, Morris JC (2004) Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. J Neuropathol Exp Neurol 63:1028–1037
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65:1863–1872
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–944
Metsaars WP, Hauw JJ, van Welsem ME, Duyckaerts C (2003) A grading system of Alzheimer disease lesions in neocortical areas. Neurobiol Aging 24:563–572
Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC (2006) [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67:446–452
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Mirra SS, Gearing M, McKeel DW Jr, Crain BJ, Hughes JP, van Belle G, Heyman A (1994) Interlaboratory comparison of neuropathology assessments in Alzheimer’s disease: a study of the consortium to establish a registry for Alzheimer’s disease (CERAD). J Neuropathol Exp Neurol 53:303–315
Morris JC (2005) Dementia update 2005. Alzheimer Dis Assoc Disord 19:100–117
Morrison JH, Hof PR (2007) Life and death of neurons in the aging cerebral cortex. Int Rev Neurobiol 81:41–57
Munoz DG, Dickson DW, Bergeron C, Mackenzie IR, Delacourte A, Zhukareva V (2003) The neuropathology and biochemistry of frontotemporal dementia. Ann Neurol 54(Suppl 5):S24–S28
Murray ME, Knopman DS, Dickson DW (2007) Vascular dementia: clinical, neuroradiologic and neuropathologic aspects. Panminerva Med 49:197–207
Nagy Z, Esiri MM, Jobst KA, Morris JH, King E-F, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD (1997) The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56:165–170
Nagy Z, Vatter-Bittner B, Braak H, Braak E, Yilmazer DM, Schultz C, Hanke J (1997) Staging of Alzheimer-type pathology: an interrater-intrarater study. Dement Geriatr Cogn Disord 8:248–251
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66:1136–1146
Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR (2008) Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol [Epub ahead of print]
Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET (1999) Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 58:1147–1155
Nolan KA, Lino MM, Seligmann AW, Blass JP (1998) Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc 46:597–604
O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001) Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 57:632–638
Pantoni L, Sarti C, Alafuzoff I, Jellinger K, Munoz DG, Ogata J, Palumbo V (2006) Postmortem examination of vascular lesions in cognitive impairment: a survey among neuropathological services. Stroke 37:1005–1009
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407
Peng FC (2003) Is dementia a disease? Gerontology 49:384–391
Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesberry W, Davis DG, Nelson J, Hardman J, Masaki KH, Vogt MR, Launer LJ, White LR (2005) AD lesions and infarcts in demented and no-demented Japanese-American men. Ann Neurol 57:98–103
Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, Kainulainen K, Kontula K, Perez-Tur J, Hardy J, Haltia M (2001) Prevalence of Alzheimer’s disease in very elderly people: a prospective neuropathological study. Neurology 56:1690–1696
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368
Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V (2006) Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology 66:49–55
Ranginwala NA, Hynan LS, Weiner MF, White CL 3rd (2008) Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry 16:384–388
Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 7:103–117 discussion 173–180
Riley KP, Snowdon DA, Markesbery WR (2002) Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the nun study. Ann Neurol 51:567–577
Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A et al (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43:250–260
Román GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, Lopez-Pousa S, Arizaga R, Wallin A (2004) Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci 226:81–87
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL (2007) Imaging betaamyloid burden in aging and dementia. Neurology 68:1718–1725
Scheff SW, Price DA (2003) Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging 24:1029–1046
Scheff SW, Price DA (2006) Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis 9:101–115
Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR (2000) “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology 55:370–376
Schnaider Beeri M, Silverman JM, Schmeidler J, Wysocki M, Grossman HZ, Purohit DP, Perl DP, Haroutunian V (2008) Clinical dementia rating performed several years prior to death predicts regional Alzheimer’s neuropathology. Dement Geriatr Cogn Disord 25:392–398
Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62:1148–1155
Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69:2197–2204
Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA (2007) Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol 62:59–66
Shin J, Lee SY, Kim SH, Kim YB, Cho SJ (2008) Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. Neuroimage 43:236–244
Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT (2002) Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med 64:493–501
Snowdon DA (1997) Aging and Alzheimer’s disease: lessons from the nun study. Gerontologist 37:150–156
Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ (2007) Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62:406–413
Terry RD, Hansen LA, DeTeresa R, Davies P, Tobias H, Katzman R (1987) Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol 46:262–268
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580
Terry RD (2000) Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol 59:1118–1119
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thal DR, Griffin WST, Braak H (2008) Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J Cell Mol Med 12:1–15
Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J (2004) The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62:1984–1989
Tiraboschi P, Sabbagh MN, Hansen LA, Salmon DP, Merdes A, Gamst A, Masliah E, Alford M, Thal LJ, Corey-Bloom J (2004) Alzheimer disease without neocortical neurofibrillary tangles: “a second look”. Neurology 62:1141–1147
Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ (2008) Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol 64:168–176
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006) Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
Uitti RJ, Calne DB, Dickson DW, Wszolek ZK (2004) Is the neuropathological ‘gold standard’ diagnosis dead? Implications of clinicopathological findings in an autosomal dominant neurodegenerative disorder. Parkinsonism Relat Disord 10:461–463
van der Zee J, Sleegers K, Van Broeckhoven C (2008) Invited article: the Alzheimer disease-frontotemporal lobar degeneration spectrum. Neurology 71:1191–1197
von Gunten A, Kovari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P (2005) Stereologic analysis of hippocampal Alzheimer’s disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol 193:198–206
von Gunten A, Bouras C, Kovari E, Giannakopoulos P, Hof PR (2006) Neural substrates of cognitive and behavioral deficits in atypical Alzheimer’s disease. Brain Res Rev 51:176–211
Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T (2007) Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp 28:967–978
Warren JD, Schott JM, Fox NC, Thom M, Revesz T, Holton JL, Scaravilli F, Thomas DG, Plant GT, Rudge P, Rossor MN (2005) Brain biopsy in dementia. Brain 128:2016–2025
Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, Vemuri P, Senjem ML, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR Jr (2008) MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71:743–749
Williamson JB, Nyenhuis DL, Pedelty L, Byrd S, Jhaveri M, Wang C, deToledo-Morrell L, Sripathirathan K, Gorelick P (2008) Baseline differences between vascular cognitive impairment no dementia reverters and non-reverters. J Neurol Neurosurg Psychiatry 79:1208–1214
Wisniewski HM, Robe A, Zigman W, Silverman W (1989) Neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 48:606–609
Woodhouse A, Shepherd CE, Sokolova A, Carroll VL, King AE, Halliday GM, Dickson TC, Vickers JC (2009) Cytoskeletal alterations differentiate presenilin-1 and sporadic Alzheimer’s disease. Acta Neuropathol 117:19–29
Xuereb JH, Brayne C, Dufouil C, Gertz H, Wischik C, Harrington C, Mukaetova-Ladinska E, McGee MA, O’Sullivan A, O’Connor D, Paykel ES, Huppert FA (2000) Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann N Y Acad Sci 903:490–496
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG (2008) Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology 70:1042–1048
Zekry D, Duyckaerts C, Belmin J, Geoffre C, Herrmann F, Moulias R, Hauw JJ (2003) The vascular lesions in vascular and mixed dementia: the weight of functional neuroanatomy. Neurobiol Aging 24:213–219
Acknowledgments
The study was supported in part by the Society for the Support of Research in Experimental Neurology, Vienna, Austria. The author thanks Mrs. V. Rappelsberger for excellent laboratory work and Mr. E. Mitter-Ferstl, PhD, for secretarial and computer work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp?. Acta Neuropathol 117, 101–110 (2009). https://doi.org/10.1007/s00401-008-0466-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0466-z